Low density lipoprotein receptor related protein 6 (LRP6) protects heart against oxidative stress by the crosstalk of HSF1 and GSK3β
- PMID: 32905882
- PMCID: PMC7486456
- DOI: 10.1016/j.redox.2020.101699
Low density lipoprotein receptor related protein 6 (LRP6) protects heart against oxidative stress by the crosstalk of HSF1 and GSK3β
Erratum in
-
Corrigendum to "Low density lipoprotein receptor related protein 6 (LRP6) protects heart against oxidative stress by the crosstalk of HSF1 and GSK3β" [Redox Biol. 37 (2020) 101699].Redox Biol. 2023 Dec;68:102954. doi: 10.1016/j.redox.2023.102954. Epub 2023 Nov 10. Redox Biol. 2023. PMID: 37949687 Free PMC article. No abstract available.
Abstract
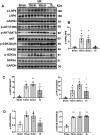
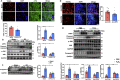
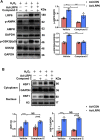
Low density lipoprotein receptor-related protein 6 (LRP6), a Wnt co-receptor, induces multiple functions in various organs. We recently reported cardiac specific LRP6 deficiency caused cardiac dysfunction in mice. Whether cardiomyocyte-expressed LRP6 protects hearts against ischemic stress is largely unknown. Here, we investigated the effects of cardiac LRP6 in response to ischemic reperfusion (I/R) injury. Tamoxifen inducible cardiac specific LRP6 overexpression mice were generated to build I/R model by occlusion of the left anterior descending (LAD) coronary artery for 40 min and subsequent release of specific time. Cardiac specific LRP6 overexpression significantly ameliorated myocardial I/R injury as characterized by the improved cardiac function, strain pattern and infarct area at 24 h after reperfusion. I/R induced-apoptosis and endoplasmic reticulum (ER) stress were greatly inhibited by LRP6 overexpression in cardiomyocytes. LRP6 overexpression enhanced the expression of heat shock transcription factor-1(HSF1) and heat shock proteins (HSPs), the level of p-glycogen synthase kinase 3β(GSK3β)(S9) and p-AMPK under I/R. HSF1 inhibitor deteriorated the apoptosis and decreased p-GSK3β(S9) level in LRP6 overexpressed -cardiomyocytes treated with H2O2. Si-HSF1 or overexpression of active GSK3β significantly attenuated the increased expression of HSF1 and p-AMPK, and the inhibition of apoptosis and ER stress induced by LRP6 overexpression in H2O2-treated cardiomyocytes. AMPK inhibitor suppressed the increase in p-GSK3β (S9) level but didn't alter HSF1 nucleus expression in LRP6 overexpressed-cardiomyocytes treated with H2O2. Active GSK3β, but not AMPK inhibitor, attenuated the inhibition of ubiquitination of HSF1 induced by LRP6-overexpressed-cardiomyocytes treated with H2O2. LRP6 overexpression increased interaction of HSF1 and GSK3β which may be involved in the reciprocal regulation under oxidative stress. In conclusion, cardiac LRP6 overexpression significantly inhibits cardiomyocyte apoptosis and ameliorates myocardial I/R injury by the crosstalk of HSF1 and GSK3β signaling.
Keywords: Apoptosis; GSK3β; HSF1; Ischemic reperfusion; LRP6.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
We declare that there are no competing interests.
Figures







References
-
- Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. - PubMed
-
- Abbate A., Narula J. Role of apoptosis in adverse ventricular remodeling. Heart Fail. Clin. 2012;8:79–86. - PubMed
-
- Wang Y., Yin C., Chen Z., Li Y., Zou Y., Wang X. Cardiac-specific LRP6 knockout induces lipid accumulation through Drp1/CPT1b pathway in adult mice. Cell Tissue Res. 2020;380:143–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

