The "Sick-but-not-Dead" Phenomenon Applied to Catecholamine Deficiency in Neurodegenerative Diseases
- PMID: 32906170
- PMCID: PMC10680399
- DOI: 10.1055/s-0040-1713874
The "Sick-but-not-Dead" Phenomenon Applied to Catecholamine Deficiency in Neurodegenerative Diseases
Abstract
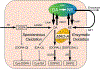
The catecholamines dopamine and norepinephrine are key central neurotransmitters that participate in many neurobehavioral processes and disease states. Norepinephrine is also the main neurotransmitter mediating regulation of the circulation by the sympathetic nervous system. Several neurodegenerative disorders feature catecholamine deficiency. The most common is Parkinson's disease (PD), in which putamen dopamine content is drastically reduced. PD also entails severely decreased myocardial norepinephrine content, a feature that characterizes two other Lewy body diseases-pure autonomic failure and dementia with Lewy bodies. It is widely presumed that tissue catecholamine depletion in these conditions results directly from loss of catecholaminergic neurons; however, as highlighted in this review, there are also important functional abnormalities in extant residual catecholaminergic neurons. We refer to this as the "sick-but-not-dead" phenomenon. The malfunctions include diminished dopamine biosynthesis via tyrosine hydroxylase (TH) and L-aromatic-amino-acid decarboxylase (LAAAD), inefficient vesicular sequestration of cytoplasmic catecholamines, and attenuated neuronal reuptake via cell membrane catecholamine transporters. A unifying explanation for catecholaminergic neurodegeneration is autotoxicity exerted by 3,4-dihydroxyphenylacetaldehyde (DOPAL), an obligate intermediate in cytoplasmic dopamine metabolism. In PD, putamen DOPAL is built up with respect to dopamine, associated with a vesicular storage defect and decreased aldehyde dehydrogenase activity. Probably via spontaneous oxidation, DOPAL potently oligomerizes and forms quinone-protein adducts with ("quinonizes") α-synuclein (AS), a major constituent in Lewy bodies, and DOPAL-induced AS oligomers impede vesicular storage. DOPAL also quinonizes numerous intracellular proteins and inhibits enzymatic activities of TH and LAAAD. Treatments targeting DOPAL formation and oxidation therefore might rescue sick-but-not-dead catecholaminergic neurons in Lewy body diseases.
Thieme. All rights reserved.
Conflict of interest statement
None.
Figures




Similar articles
-
The heart of PD: Lewy body diseases as neurocardiologic disorders.Brain Res. 2019 Jan 1;1702:74-84. doi: 10.1016/j.brainres.2017.09.033. Epub 2017 Oct 10. Brain Res. 2019. PMID: 29030055 Free PMC article. Review.
-
"Sick-but-not-dead": multiple paths to catecholamine deficiency in Lewy body diseases.Stress. 2020 Nov;23(6):633-637. doi: 10.1080/10253890.2020.1765158. Epub 2020 May 25. Stress. 2020. PMID: 32372682 Free PMC article. Review.
-
The catecholaldehyde hypothesis: where MAO fits in.J Neural Transm (Vienna). 2020 Feb;127(2):169-177. doi: 10.1007/s00702-019-02106-9. Epub 2019 Dec 5. J Neural Transm (Vienna). 2020. PMID: 31807952 Free PMC article. Review.
-
Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders.Pharmacol Ther. 2014 Dec;144(3):268-82. doi: 10.1016/j.pharmthera.2014.06.006. Epub 2014 Jun 16. Pharmacol Ther. 2014. PMID: 24945828 Free PMC article. Review.
-
Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy.Parkinsonism Relat Disord. 2015 Jun;21(6):567-72. doi: 10.1016/j.parkreldis.2015.03.006. Epub 2015 Mar 20. Parkinsonism Relat Disord. 2015. PMID: 25829070 Free PMC article.
Cited by
-
Could Small Heat Shock Protein HSP27 Be a First-Line Target for Preventing Protein Aggregation in Parkinson's Disease?Int J Mol Sci. 2021 Mar 16;22(6):3038. doi: 10.3390/ijms22063038. Int J Mol Sci. 2021. PMID: 33809767 Free PMC article. Review.
-
Natural Compounds That Activate the KEAP1/Nrf2 Signaling Pathway as Potential New Drugs in the Treatment of Idiopathic Parkinson's Disease.Antioxidants (Basel). 2024 Sep 18;13(9):1125. doi: 10.3390/antiox13091125. Antioxidants (Basel). 2024. PMID: 39334784 Free PMC article. Review.
-
Cardiac sympathetic burden reflects Parkinson disease burden, regardless of high or low orthostatic blood pressure changes.NPJ Parkinsons Dis. 2021 Aug 12;7(1):71. doi: 10.1038/s41531-021-00217-3. NPJ Parkinsons Dis. 2021. PMID: 34385459 Free PMC article.
-
Targets to Search for New Pharmacological Treatment in Idiopathic Parkinson's Disease According to the Single-Neuron Degeneration Model.Biomolecules. 2024 Jun 8;14(6):673. doi: 10.3390/biom14060673. Biomolecules. 2024. PMID: 38927076 Free PMC article. Review.
-
Astrocytes protect dopaminergic neurons against aminochrome neurotoxicity.Neural Regen Res. 2022 Sep;17(9):1861-1866. doi: 10.4103/1673-5374.335690. Neural Regen Res. 2022. PMID: 35142659 Free PMC article. Review.
References
-
- Goldstein DS, Eisenhofer G, McCarty R. Catecholamines: Bridging Basic Science with Clinical Medicine. New York: Academic Press; 1998
-
- Nagatsu T, Nabeshima T, Goldstein DS, eds. Catecholamine Research: From Molecular Insights to Clinical Medicine. New York, NY: Plenum; 2002
-
- Eiden LE. A New Era of Catecholamines in the Laboratory and Clinic. In: Eiden LE ed. New York: Elsevier; 2013
-
- Esler M The sympathetic nervous system through the ages: from Thomas Willis to resistant hypertension. Exp Physiol 2011;96 (07):611–622 - PubMed

