Therapeutically Significant MicroRNAs in Primary and Metastatic Brain Malignancies
- PMID: 32906592
- PMCID: PMC7564168
- DOI: 10.3390/cancers12092534
Therapeutically Significant MicroRNAs in Primary and Metastatic Brain Malignancies
Abstract
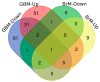
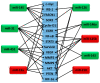
Brain cancer is one among the rare cancers with high mortality rate that affects both children and adults. The most aggressive form of primary brain tumor is glioblastoma. Secondary brain tumors most commonly metastasize from primary cancers of lung, breast, or melanoma. The five-year survival of primary and secondary brain tumors is 34% and 2.4%, respectively. Owing to poor prognosis, tumor heterogeneity, increased tumor relapse, and resistance to therapies, brain cancers have high mortality and poor survival rates compared to other cancers. Early diagnosis, effective targeted treatments, and improved prognosis have the potential to increase the survival rate of patients with primary and secondary brain malignancies. MicroRNAs (miRNAs) are short noncoding RNAs of approximately 18-22 nucleotides that play a significant role in the regulation of multiple genes. With growing interest in the development of miRNA-based therapeutics, it is crucial to understand the differential role of these miRNAs in the given cancer scenario. This review focuses on the differential expression of ten miRNAs (miR-145, miR-31, miR-451, miR-19a, miR-143, miR-125b, miR-328, miR-210, miR-146a, and miR-126) in glioblastoma and brain metastasis. These miRNAs are highly dysregulated in both primary and metastatic brain tumors, which necessitates a better understanding of their role in these cancers. In the context of the tumor microenvironment and the expression of different genes, these miRNAs possess both oncogenic and/or tumor-suppressive roles within the same cancer.
Keywords: brain cancer; cancer metastasis; glioblastoma; glioma; miRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

