A Series of Tubes: The C. elegans Excretory Canal Cell as a Model for Tubule Development
- PMID: 32906663
- PMCID: PMC7557474
- DOI: 10.3390/jdb8030017
A Series of Tubes: The C. elegans Excretory Canal Cell as a Model for Tubule Development
Abstract
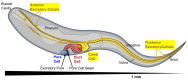
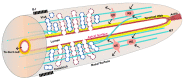
Formation and regulation of properly sized epithelial tubes is essential for multicellular life. The excretory canal cell of C. elegans provides a powerful model for investigating the integration of the cytoskeleton, intracellular transport, and organismal physiology to regulate the developmental processes of tube extension, lumen formation, and lumen diameter regulation in a narrow single cell. Multiple studies have provided new understanding of actin and intermediate filament cytoskeletal elements, vesicle transport, and the role of vacuolar ATPase in determining tube size. Most of the genes discovered have clear homologues in humans, with implications for understanding these processes in mammalian tissues such as Schwann cells, renal tubules, and brain vasculature. The results of several new genetic screens are described that provide a host of new targets for future studies in this informative structure.
Keywords: IRG protein; apical surface; epithelial tube; exocytosis; intermediate filaments; terminal web; vacuolar ATPase; vesicle transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Samakovlis C., Hacohen N., Manning G., Sutherland D.C., Guillemin K., Krasnow M.A. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

