Non-Hematologic Toxicity of Bortezomib in Multiple Myeloma: The Neuromuscular and Cardiovascular Adverse Effects
- PMID: 32906684
- PMCID: PMC7563977
- DOI: 10.3390/cancers12092540
Non-Hematologic Toxicity of Bortezomib in Multiple Myeloma: The Neuromuscular and Cardiovascular Adverse Effects
Abstract
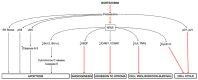
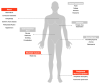
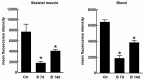
The overall approach to the treatment of multiple myeloma (MM) has undergone several changes during the past decade. and proteasome inhibitors (PIs) including bortezomib, carfilzomib, and ixazomib have considerably improved the outcomes in affected patients. The first-in-class selective PI bortezomib has been initially approved for the refractory forms of the disease but has now become, in combination with other drugs, the backbone of the frontline therapy for newly diagnosed MM patients, as well as in the maintenance therapy and relapsed/refractory setting. Despite being among the most widely used and highly effective agents for MM, bortezomib can induce adverse events that potentially lead to early discontinuation of the therapy with negative effects on the quality of life and outcome of the patients. Although peripheral neuropathy and myelosuppression have been recognized as the most relevant bortezomib-related adverse effects, cardiac and skeletal muscle toxicities are relatively common in MM treated patients, but they have received much less attention. Here we review the neuromuscular and cardiovascular side effects of bortezomib. focusing on the molecular mechanisms underlying its toxicity. We also discuss our preliminary data on the effects of bortezomib on skeletal muscle tissue in mice receiving the drug.
Keywords: bortezomib; cardiotoxicity; multiple myeloma; muscle toxicity; peripheral neuropathy; proteasome inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.-V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
-
- Landgren O., Kyle R.A., Pfeiffer R.M., Katzmann J.A., Caporaso N.E., Hayes R.B., Dispenzieri A., Kumar S., Clark R.J., Baris D., et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. - DOI - PMC - PubMed
-
- Howlader N., Noone A.M., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., et al. SEER Cancer Statistics Review, 1975–2017. National Cancer Institute; Bethesda, MD, USA: 2020.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

