The Emerging Relevance of AIM2 in Liver Disease
- PMID: 32906750
- PMCID: PMC7555176
- DOI: 10.3390/ijms21186535
The Emerging Relevance of AIM2 in Liver Disease
Abstract
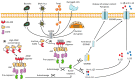
Absent in melanoma 2 (AIM2) is a cytosolic receptor that recognizes double-stranded DNA (dsDNA) and triggers the activation of the inflammasome cascade. Activation of the inflammasome results in the maturation of inflammatory cytokines, such as interleukin (IL)-1 β and IL-18, and a form of cell death known as pyroptosis. Owing to the conserved nature of its ligand, AIM2 is important during immune recognition of multiple pathogens. Additionally, AIM2 is also capable of recognizing host DNA during cellular damage or stress, thereby contributing to sterile inflammatory diseases. Inflammation, either in response to pathogens or due to sterile cellular damage, is at the center of the most prevalent and life-threatening liver diseases. Therefore, during the last 15 years, the study of inflammasome activation in the liver has emerged as a new research area in hepatology. Here, we discuss the known functions of AIM2 in the pathogenesis of different hepatic diseases, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), hepatitis B, liver fibrosis, and hepatocellular carcinoma (HCC).
Keywords: AIM2; HCC; NAFLD; NASH; absent in melanoma 2; cirrhosis; fibrosis; hepatitis; inflammasome; liver disease.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
A novel role of AIM2 inflammasome-mediated pyroptosis in radiofrequency ablation of hepatocellular carcinoma.Ann Hepatol. 2024 Nov-Dec;29(6):101532. doi: 10.1016/j.aohep.2024.101532. Epub 2024 Jul 22. Ann Hepatol. 2024. PMID: 39048057
-
AIM2 deficiency reduces the development of hepatocellular carcinoma in mice.Int J Cancer. 2018 Dec 1;143(11):2997-3007. doi: 10.1002/ijc.31827. Epub 2018 Oct 9. Int J Cancer. 2018. PMID: 30133699
-
The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury.Brain Res. 2018 Oct 15;1697:10-20. doi: 10.1016/j.brainres.2018.06.008. Epub 2018 Jun 8. Brain Res. 2018. PMID: 29886252
-
Role of AIM2 inflammasome in inflammatory diseases, cancer and infection.Eur J Immunol. 2019 Nov;49(11):1998-2011. doi: 10.1002/eji.201848070. Epub 2019 Aug 14. Eur J Immunol. 2019. PMID: 31372985 Free PMC article. Review.
-
The emerging roles of absent in melanoma 2 (AIM2) inflammasome in central nervous system disorders.Neurochem Int. 2021 Oct;149:105122. doi: 10.1016/j.neuint.2021.105122. Epub 2021 Jul 17. Neurochem Int. 2021. PMID: 34284076 Review.
Cited by
-
Hepatocellular carcinoma and AIM2: Therapeutic potential through regulation of autophagy and macrophage polarization.Immun Inflamm Dis. 2024 Sep;12(9):e70002. doi: 10.1002/iid3.70002. Immun Inflamm Dis. 2024. PMID: 39222064 Free PMC article.
-
AIM2 fosters lung adenocarcinoma immune escape by modulating PD-L1 expression in tumor-associated macrophages via JAK/STAT3.Hum Vaccin Immunother. 2023 Dec 15;19(3):2269790. doi: 10.1080/21645515.2023.2269790. Epub 2023 Oct 25. Hum Vaccin Immunother. 2023. PMID: 37877820 Free PMC article.
-
Cytoplasmic DNA and AIM2 inflammasome in RA: where they come from and where they go?Front Immunol. 2024 Oct 10;15:1343325. doi: 10.3389/fimmu.2024.1343325. eCollection 2024. Front Immunol. 2024. PMID: 39450183 Free PMC article. Review.
-
Identification and validation of a novel pyroptosis-related lncRNAs signature associated with prognosis and immune regulation of hepatocellular carcinoma.Sci Rep. 2022 May 25;12(1):8886. doi: 10.1038/s41598-022-13046-y. Sci Rep. 2022. PMID: 35614201 Free PMC article.
-
The Role of the AIM2 Gene in Obesity-Related Glucose and Lipid Metabolic Disorders: A Recent Update.Diabetes Metab Syndr Obes. 2024 Oct 21;17:3903-3916. doi: 10.2147/DMSO.S488978. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39465122 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

