Eosinophils and Neutrophils-Molecular Differences Revealed by Spontaneous Raman, CARS and Fluorescence Microscopy
- PMID: 32906767
- PMCID: PMC7563840
- DOI: 10.3390/cells9092041
Eosinophils and Neutrophils-Molecular Differences Revealed by Spontaneous Raman, CARS and Fluorescence Microscopy
Abstract
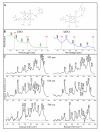
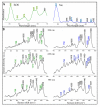
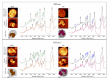
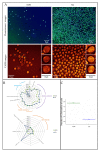
Leukocytes are a part of the immune system that plays an important role in the host's defense against viral, bacterial, and fungal infections. Among the human leukocytes, two granulocytes, neutrophils (Ne) and eosinophils (EOS) play an important role in the innate immune system. For that purpose, eosinophils and neutrophils contain specific granules containing protoporphyrin-type proteins such as eosinophil peroxidase (EPO) and myeloperoxidase (MPO), respectively, which contribute directly to their anti-infection activity. Since both proteins are structurally and functionally different, they could potentially be a marker of both cells' types. To prove this hypothesis, UV-Vis absorption spectroscopy and Raman imaging were applied to analyze EPO and MPO and their content in leukocytes isolated from the whole blood. Moreover, leukocytes can contain lipidic structures, called lipid bodies (LBs), which are linked to the regulation of immune responses and are considered to be a marker of cell inflammation. In this work, we showed how to determine the number of LBs in two types of granulocytes, EOS and Ne, using fluorescence and coherent anti-Stokes Raman scattering (CARS) microscopy. Spectroscopic differences of EPO and MPO can be used to identify these cells in blood samples, while the detection of LBs can indicate the cell inflammation process.
Keywords: Raman microscopy; coherent anti-Stokes Raman scattering (CARS), fluorescence microscopy; eosinophil peroxidase; eosinophils; lipid bodies; myeloperoxidase; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Maton A., Lahart D., Hopkins J., Warner M.Q., Johnson S., Wright J.D. Cells: Building Blocks of Life. 3rd ed. Prentice Hall science; Pearson Prentice Hall; Upper Saddle River, NJ, USA: 1997.
-
- Giembycz M.A., Lindsay M.A. Pharmacology of the Eosinophil. Pharmacol. Rev. 1999;51:213–340. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

