Standardization and Validation of Fluorescence-Based Quantitative Assay to Study Human Platelet Adhesion to Extracellular-Matrix in a 384-Well Plate
- PMID: 32906775
- PMCID: PMC7554887
- DOI: 10.3390/ijms21186539
Standardization and Validation of Fluorescence-Based Quantitative Assay to Study Human Platelet Adhesion to Extracellular-Matrix in a 384-Well Plate
Abstract
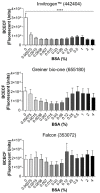
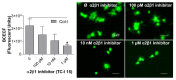
Platelets play a crucial role in the immunological response and are involved in the pathological settings of vascular diseases, and their adhesion to the extracellular matrix is important to bring leukocytes close to the endothelial cells and to form and stabilize the thrombus. Currently there are several methods to study platelet adhesion; however, the optimal parameters to perform the assay vary among studies, which hinders their comparison and reproducibility. Here, a standardization and validation of a fluorescence-based quantitative adhesion assay to study platelet-ECM interaction in a high-throughput screening format is proposed. Our study confirms that fluorescence-based quantitative assays can be effectively used to detect platelet adhesion, in which BCECF-AM presents the highest sensitivity in comparison to other dyes.
Keywords: 384-well plate; BCECF-AM; extracellular matrix; fluorescence-based quantitative assay; high-throughput screening assay; platelet adhesion.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Frenette P.S., Denis C.V., Weiss L., Jurk K., Subbarao S., Kehrel B., Hartwig J.H., Vestweber D., Wagner D.D. P-Selectin Glycoprotein Ligand 1 (Psgl-1) Is Expressed on Platelets and Can Mediate Platelet–Endothelial Interactions in Vivo. J. Exp. Med. 2000;191:1413–1422. doi: 10.1084/jem.191.8.1413. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

