Large Extracellular Vesicles-A New Frontier of Liquid Biopsy in Oncology
- PMID: 32906787
- PMCID: PMC7555129
- DOI: 10.3390/ijms21186543
Large Extracellular Vesicles-A New Frontier of Liquid Biopsy in Oncology
Abstract
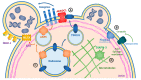
Extracellular Vesicles (EVs) are emerging as pivotal elements in cancer. Many studies have focused on the role of Small- (S)-EVs but in recent years Large-(L)-EVs have progressively gained increasing interest due to their peculiar content and functions. Tumor-derived L-EVs carry a lot of oncogenic proteins, nucleic acids and lipids to recipient cells and are involved in the reshaping of the tumor microenvironment as well as in the metabolic rewiring and the promotion of the pro-metastatic attitude of cancer cells. Several techniques have been developed for the isolation of L-EVs and commercial kits are also available for efficient and easy recovery of these vesicles. Also, the improvement in DNA sequencing and "omics sciences" profoundly changed the way to analyze and explore the molecular content of L-EVs, thus providing novel and potentially useful cancer biomarkers. Herein, we review the most recent findings concerning the role of L-EVs in cancer and discuss their possible use in oncology as "liquid biopsy" tools as compared to the other classes of EVs.
Keywords: cancer; exosomes; large extracellular vesicles; microvesicles; oncosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Extracellular Vesicles: A Brief Overview and Its Role in Precision Medicine.Methods Mol Biol. 2017;1660:1-14. doi: 10.1007/978-1-4939-7253-1_1. Methods Mol Biol. 2017. PMID: 28828643 Review.
-
Extracellular Vesicles: Recent Developments in Technology and Perspectives for Cancer Liquid Biopsy.Recent Results Cancer Res. 2020;215:319-344. doi: 10.1007/978-3-030-26439-0_17. Recent Results Cancer Res. 2020. PMID: 31605237 Review.
-
Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance.Drug Resist Updat. 2019 Dec;47:100647. doi: 10.1016/j.drup.2019.100647. Epub 2019 Oct 15. Drug Resist Updat. 2019. PMID: 31704541 Review.
-
Metabolome of Exosomes: Focus on Vesicles Released by Cancer Cells and Present in Human Body Fluids.Int J Mol Sci. 2019 Jul 14;20(14):3461. doi: 10.3390/ijms20143461. Int J Mol Sci. 2019. PMID: 31337156 Free PMC article. Review.
-
Extracellular vesicles compartment in liquid biopsies: Clinical application.Mol Aspects Med. 2018 Apr;60:27-37. doi: 10.1016/j.mam.2017.11.009. Epub 2017 Nov 26. Mol Aspects Med. 2018. PMID: 29155161 Review.
Cited by
-
Cell-cell contact-dependent secretion of large-extracellular vesicles from EFNBhigh cancer cells accelerates peritoneal dissemination.Br J Cancer. 2024 Oct;131(6):982-995. doi: 10.1038/s41416-024-02783-8. Epub 2024 Jul 13. Br J Cancer. 2024. PMID: 39003372 Free PMC article.
-
Distinguishing functional exosomes and other extracellular vesicles as a nucleic acid cargo by the anion-exchange method.J Extracell Vesicles. 2022 Mar;11(3):e12205. doi: 10.1002/jev2.12205. J Extracell Vesicles. 2022. PMID: 35289089 Free PMC article.
-
Extracellular Vesicles in Advanced Prostate Cancer: Tools to Predict and Thwart Therapeutic Resistance.Cancers (Basel). 2021 Jul 28;13(15):3791. doi: 10.3390/cancers13153791. Cancers (Basel). 2021. PMID: 34359692 Free PMC article. Review.
-
Nanoprojectile Secondary Ion Mass Spectrometry Enables Multiplexed Analysis of Individual Hepatic Extracellular Vesicles.ACS Nano. 2023 Dec 12;17(23):23584-23594. doi: 10.1021/acsnano.3c06604. Epub 2023 Nov 30. ACS Nano. 2023. PMID: 38033295 Free PMC article.
-
Lysosomes in Cancer-At the Crossroad of Good and Evil.Cells. 2024 Mar 5;13(5):459. doi: 10.3390/cells13050459. Cells. 2024. PMID: 38474423 Free PMC article. Review.
References
-
- Minciacchi V.R., You S., Spinelli C., Morley S., Zandian M., Aspuria P.J., Cavallini L., Ciardiello C., Reis Sobreiro M., Morello M., et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327–11341. doi: 10.18632/oncotarget.3598. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

