Cholestasis Differentially Affects Liver Connexins
- PMID: 32906817
- PMCID: PMC7116118
- DOI: 10.3390/ijms21186534
Cholestasis Differentially Affects Liver Connexins
Abstract
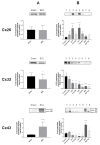
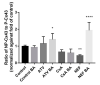
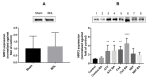
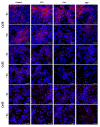
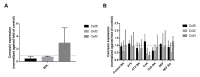
Connexins are goal keepers of tissue homeostasis, including in the liver. As a result, they are frequently involved in disease. The current study was set up to investigate the effects of cholestatic disease on the production of connexin26, connexin32 and connexin43 in the liver. For this purpose, bile duct ligation, a well-known trigger of cholestatic liver injury, was applied to mice. In parallel, human hepatoma HepaRG cell cultures were exposed to cholestatic drugs and bile acids. Samples from both the in vivo and in vitro settings were subsequently subjected to assessment of mRNA and protein quantities as well as to in situ immunostaining. While the outcome of cholestasis on connexin26 and connexin43 varied among experimental settings, a more generalized repressing effect was seen for connexin32. This has also been observed in many other liver pathologies and could suggest a role for connexin32 as a robust biomarker of liver disease and toxicity.
Keywords: cholestasis; connexin; liver.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Expression and Functionality of Connexin-Based Channels in Human Liver Cancer Cell Lines.Int J Mol Sci. 2021 Nov 10;22(22):12187. doi: 10.3390/ijms222212187. Int J Mol Sci. 2021. PMID: 34830068 Free PMC article.
-
Potential of connexin 32 as a predictive marker for drug-induced cholestatic liver injury in a collagen vitrigel-culture model of HepG2-NIAS cells, a new subline of HepG2 cells, with bile canaliculus-like structures.J Toxicol Sci. 2025;50(3):135-145. doi: 10.2131/jts.50.135. J Toxicol Sci. 2025. PMID: 40024757
-
Serotonin protects mouse liver from cholestatic injury by decreasing bile salt pool after bile duct ligation.Hepatology. 2012 Jul;56(1):209-18. doi: 10.1002/hep.25626. Epub 2012 Jun 5. Hepatology. 2012. PMID: 22290718
-
Bile salts and cholestasis.Dig Liver Dis. 2010 Jun;42(6):409-18. doi: 10.1016/j.dld.2010.03.015. Dig Liver Dis. 2010. PMID: 20434968 Review.
-
Bile acid transporters: structure, function, regulation and pathophysiological implications.Pharm Res. 2007 Oct;24(10):1803-23. doi: 10.1007/s11095-007-9289-1. Epub 2007 Apr 3. Pharm Res. 2007. PMID: 17404808 Review.
Cited by
-
Expression and Functionality of Connexin-Based Channels in Human Liver Cancer Cell Lines.Int J Mol Sci. 2021 Nov 10;22(22):12187. doi: 10.3390/ijms222212187. Int J Mol Sci. 2021. PMID: 34830068 Free PMC article.
-
Connexin-Based Channel Activity Is Not Specifically Altered by Hepatocarcinogenic Chemicals.Int J Mol Sci. 2021 Oct 29;22(21):11724. doi: 10.3390/ijms222111724. Int J Mol Sci. 2021. PMID: 34769157 Free PMC article.
-
Impact of partial bile duct ligation with or without repeated magnetic resonance imaging examinations in mice.Sci Rep. 2022 Dec 5;12(1):21014. doi: 10.1038/s41598-022-25318-8. Sci Rep. 2022. PMID: 36470922 Free PMC article.
-
The altered osteocytic expression of connexin 43 and sclerostin in human cadaveric donors with alcoholic liver cirrhosis: Potential treatment targets.J Anat. 2022 Jun;240(6):1162-1173. doi: 10.1111/joa.13621. Epub 2022 Jan 3. J Anat. 2022. PMID: 34978341 Free PMC article.
-
Blocking connexin 43 and its promotion of ATP release from renal tubular epithelial cells ameliorates renal fibrosis.Cell Death Dis. 2022 May 31;13(5):511. doi: 10.1038/s41419-022-04910-w. Cell Death Dis. 2022. PMID: 35641484 Free PMC article.
References
-
- Neveu M.J., Babcock K.L., Hertzberg E.L., Paul D.L., Nicholson B.J., Pitot H.C. Colocalized Alterations in connexin32 and Cytochrome P450IIB1/2 by Phenobarbital and Related Liver Tumor Promoters. Cancer Res. 1994;54:3145–3152. - PubMed
-
- Shoda T., Mitsumori K., Onodera H., Toyoda K., Uneyama C., Takada K., Hirose M. Live Tumor-Promoting Effect of β-Naphthoflavone, a Strong CYP 1A1/2 Inducer, and the Relationship between CYP 1A1/2 Induction and Cx32 Decrease in Its Hepatocarcinogenesis in the Rat. Toxicol. Pathol. 2000;28:540–547. doi: 10.1177/019262330002800406. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 861913/ERC_/European Research Council/International
- "Willy Gepts Fonds" UZ-VUB/University Hospital of the Vrije Universiteit Brussel-Belgium
- G009514N, G010214N and G012318N/Fonds Wetenschappelijk Onderzoek
- FAPESP SPEC grant 2013/50420-6 and FAPESP-FWO grant 18/10953-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources
Research Materials

