THRIL mediates endothelial progenitor cells autophagy via AKT pathway and FUS
- PMID: 32907536
- PMCID: PMC7488174
- DOI: 10.1186/s10020-020-00201-2
THRIL mediates endothelial progenitor cells autophagy via AKT pathway and FUS
Abstract
Background: This study focused on the roles of lncRNA THRIL in coronary atherosclerotic heart disease (CAD) through regulating AKT signaling pathway and directly interacting with FUS.
Methods: QRT-PCR was conducted to detect the expression of THRIL in CAD blood samples and endothelial progenitor cells (EPCs). Cell autophagy of EPCs was examined through Cyto-ID Autophagy Detection Kit. CCK-8 assay and flow cytometry were carried out to assess cell viability and apoptosis under various interference conditions. Western blotting was conducted to detect the expression of interest proteins. The expression levels of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) were measured by qRT-PCR. The direct interactions between HCG18 and FUS was confirmed through RNA electrophoretic mobility shift assay (RNA EMSA) and RNA immunoprecipitation (RIP) assay.
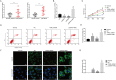
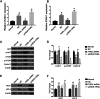
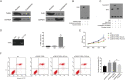
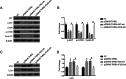
Results: THRIL was upregulated in CAD blood samples and EPCs. Knockdown of THRIL in EPCs promoted cell viability, inhibited cell autophagy and further suppressed the development of CAD. Over-expression of THRIL induced inactivation of AKT pathway, while knockdown of THRIL played reversed effects. THRIL directly interacted with FUS protein and knockdown of FUS reversed the over-expressing effect of THRIL on cell proliferation, autophagy and the status of AKT pathway.
Conclusion: THRIL inhibits the proliferation and mediates autophagy of endothelial progenitor cells via AKT pathway and FUS.
Keywords: AKT; Coronary heart disease; FUS; THRIL.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
MALAT1/miR-15b-5p/MAPK1 mediates endothelial progenitor cells autophagy and affects coronary atherosclerotic heart disease via mTOR signaling pathway.Aging (Albany NY). 2019 Feb 21;11(4):1089-1109. doi: 10.18632/aging.101766. Aging (Albany NY). 2019. PMID: 30787203 Free PMC article.
-
The long noncoding RNA THRIL knockdown protects hypoxia-induced injuries of H9C2 cells through regulating miR-99a.Cardiol J. 2019;26(5):564-574. doi: 10.5603/CJ.a2018.0054. Epub 2018 May 10. Cardiol J. 2019. PMID: 29745968 Free PMC article.
-
Long Non-coding RNA THRIL Mediates Cell Growth and Inflammatory Response of Fibroblast-Like Synoviocytes by Activating PI3K/AKT Signals in Rheumatoid Arthritis.Inflammation. 2020 Jun;43(3):1044-1053. doi: 10.1007/s10753-020-01189-x. Inflammation. 2020. PMID: 32232711
-
MiR-205 promotes endothelial progenitor cell angiogenesis and deep vein thrombosis recanalization and resolution by targeting PTEN to regulate Akt/autophagy pathway and MMP2 expression.J Cell Mol Med. 2019 Dec;23(12):8493-8504. doi: 10.1111/jcmm.14739. Epub 2019 Oct 21. J Cell Mol Med. 2019. PMID: 31633295 Free PMC article.
-
A cytoplasmic long noncoding RNA LINC00470 as a new AKT activator to mediate glioblastoma cell autophagy.J Hematol Oncol. 2018 Jun 4;11(1):77. doi: 10.1186/s13045-018-0619-z. J Hematol Oncol. 2018. PMID: 29866190 Free PMC article.
Cited by
-
Autophagy-Sirtuin1(SIRT1) Alleviated the Coronary Atherosclerosis (AS)in Mice through Regulating the Proliferation and Migration of Endothelial Progenitor Cells (EPCs) via wnt/β-catenin/GSK3β Signaling Pathway.J Nutr Health Aging. 2022;26(3):297-306. doi: 10.1007/s12603-022-1750-7. J Nutr Health Aging. 2022. PMID: 35297474 Free PMC article.
-
LncRNA THRIL Functions as a Marker for Carotid Artery Stenosis and Affects the Biological Function of Human Aortic Endothelial Cell.J Inflamm Res. 2023 Jun 8;16:2437-2446. doi: 10.2147/JIR.S409679. eCollection 2023. J Inflamm Res. 2023. PMID: 37313306 Free PMC article.
-
Regulation of Endothelial Progenitor Cell Functions in Ischemic Heart Disease: New Therapeutic Targets for Cardiac Remodeling and Repair.Front Cardiovasc Med. 2022 May 23;9:896782. doi: 10.3389/fcvm.2022.896782. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35677696 Free PMC article. Review.
-
LncRNA THRIL promotes high glucose-induced proliferation and migration of human retina microvascular endothelial cells through enhancing autophagy.Acta Diabetol. 2022 Mar;59(3):369-380. doi: 10.1007/s00592-021-01813-8. Epub 2021 Oct 30. Acta Diabetol. 2022. PMID: 34718852
-
Long non-coding RNA CATIP antisense RNA 1 (lncRNA CATIP-AS1) downregulation contributes to the progression and metastasis of thyroid cancer via epithelial-mesenchymal transition (EMT) pathway.Bioengineered. 2022 Mar;13(3):7592-7606. doi: 10.1080/21655979.2022.2047400. Bioengineered. 2022. PMID: 35264071 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

