Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis
- PMID: 32907551
- PMCID: PMC7488337
- DOI: 10.1186/s12931-020-01500-2
Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis
Abstract
Background: Ferroptosis is a new type of nonapoptotic cell death model that was closely related to reactive oxygen species (ROS) accumulation. Seawater drowning-induced acute lung injury (ALI) which is caused by severe oxidative stress injury, has been a major cause of accidental death worldwide. The latest evidences indicate nuclear factor (erythroid-derived 2)-like 2 (Nrf2) suppress ferroptosis and maintain cellular redox balance. Here, we test the hypothesis that activation of Nrf2 pathway attenuates seawater drowning-induced ALI via inhibiting ferroptosis.
Methods: we performed studies using Nrf2-specific agonist (dimethyl fumarate), Nrf2 inhibitor (ML385), Nrf2-knockout mice and ferroptosis inhibitor (Ferrostatin-1) to investigate the potential roles of Nrf2 on seawater drowning-induced ALI and the underlying mechanisms.
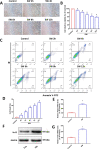
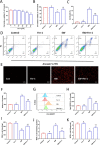
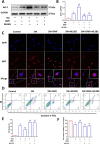
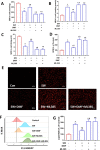
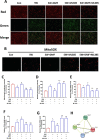
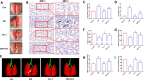
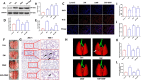
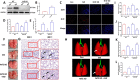
Results: Our data shows that Nrf2 activator dimethyl fumarate could increase cell viability, reduced the levels of intracellular ROS and lipid ROS, prevented glutathione depletion and lipid peroxide accumulation, increased FTH1 and GPX4 mRNA expression, and maintained mitochondrial membrane potential in MLE-12 cells. However, ML385 promoted cell death and lipid ROS production in MLE-12 cells. Furthermore, the lung injury became more aggravated in the Nrf2-knockout mice than that in WT mice after seawater drowning.
Conclusions: These results suggested that Nrf2 can inhibit ferroptosis and therefore alleviate ALI induced by seawater drowning. The effectiveness of ferroptosis inhibition by Nrf2 provides a novel therapeutic target for seawater drowning-induced ALI.
Keywords: Acute lung injury; Drowning; Ferroptosis; Nrf2; Seawater.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury.Cell Death Differ. 2020 Sep;27(9):2635-2650. doi: 10.1038/s41418-020-0528-x. Epub 2020 Mar 18. Cell Death Differ. 2020. PMID: 32203170 Free PMC article.
-
Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11.Cell Death Dis. 2021 Oct 29;12(11):1027. doi: 10.1038/s41419-021-04307-1. Cell Death Dis. 2021. PMID: 34716298 Free PMC article.
-
Nrf2 and STAT3 Alleviates Ferroptosis-Mediated IIR-ALI by Regulating SLC7A11.Oxid Med Cell Longev. 2020 Sep 18;2020:5146982. doi: 10.1155/2020/5146982. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33014271 Free PMC article.
-
Transcription factor NF-E2-related factor 2 plays a critical role in acute lung injury/acute respiratory distress syndrome (ALI/ARDS) by regulating ferroptosis.PeerJ. 2024 Jul 30;12:e17692. doi: 10.7717/peerj.17692. eCollection 2024. PeerJ. 2024. PMID: 39670103 Free PMC article. Review.
-
Astragaloside IV regulates the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to inhibit PM2.5-mediated lung injury in mice.Int Immunopharmacol. 2022 Nov;112:109186. doi: 10.1016/j.intimp.2022.109186. Epub 2022 Sep 15. Int Immunopharmacol. 2022. PMID: 36115280 Review.
Cited by
-
YAP1 protects against septic liver injury via ferroptosis resistance.Cell Biosci. 2022 Oct 1;12(1):163. doi: 10.1186/s13578-022-00902-7. Cell Biosci. 2022. PMID: 36182901 Free PMC article.
-
The role of ferroptosis in acute lung injury.Mol Cell Biochem. 2022 May;477(5):1453-1461. doi: 10.1007/s11010-021-04327-7. Epub 2022 Feb 15. Mol Cell Biochem. 2022. PMID: 35166985 Free PMC article. Review.
-
Dimethyl Fumarate Ameliorates Doxorubicin-Induced Cardiotoxicity By Activating the Nrf2 Pathway.Front Pharmacol. 2022 Apr 26;13:872057. doi: 10.3389/fphar.2022.872057. eCollection 2022. Front Pharmacol. 2022. PMID: 35559248 Free PMC article.
-
CFTR potentiator ivacaftor protects against noise-induced hair cell loss by increasing Nrf2 and reducing oxidative stress.Biomed Pharmacother. 2023 Oct;166:115399. doi: 10.1016/j.biopha.2023.115399. Epub 2023 Aug 31. Biomed Pharmacother. 2023. PMID: 37657258 Free PMC article.
-
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID.NPJ Sci Food. 2024 Mar 30;8(1):19. doi: 10.1038/s41538-024-00261-2. NPJ Sci Food. 2024. PMID: 38555403 Free PMC article. Review.
References
-
- Handley AJ. Drowning. BMJ. 2014;348:g1734. - PubMed
-
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med. 2012;366(22):2102–2110. - PubMed
-
- The L. Drowning: a silent killer. Lancet. 2017;389(10082):1859. - PubMed
-
- Gregorakos L, Markou N, Psalida V, Kanakaki M, Alexopoulou A, Sotiriou E, et al. Near-drowning: clinical course of lung injury in adults. Lung. 2009;187(2):93–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

