Chitosan-miRNA functionalized microporous titanium oxide surfaces via a layer-by-layer approach with a sustained release profile for enhanced osteogenic activity
- PMID: 32907598
- PMCID: PMC7487814
- DOI: 10.1186/s12951-020-00674-7
Chitosan-miRNA functionalized microporous titanium oxide surfaces via a layer-by-layer approach with a sustained release profile for enhanced osteogenic activity
Abstract
Background: The biofunctionalization of titanium implants for high osteogenic ability is a promising approach for the development of advanced implants to promote osseointegration, especially in compromised bone conditions. In this study, polyelectrolyte multilayers (PEMs) were fabricated using the layer-by-layer approach with a chitosan-miRNA (CS-miRNA) complex and sodium hyaluronate (HA) as the positively and negatively charged polyelectrolytes on microarc-oxidized (MAO) Ti surfaces via silane-glutaraldehyde coupling.
Methods: Dynamic contact angle and scanning electron microscopy measurements were conducted to monitor the layer accumulation. RiboGreen was used to quantify the miRNA loading and release profile in phosphate-buffered saline. The in vitro transfection efficiency and the cytotoxicity were investigated after seeding mesenchymal stem cells (MSCs) on the CS-antimiR-138/HA PEM-functionalized microporous Ti surface. The in vitro osteogenic differentiation of the MSCs and the in vivo osseointegration were also evaluated.
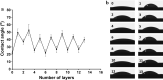
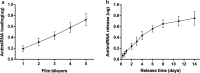
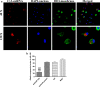
Results: The surface wettability alternately changed during the formation of PEMs. The CS-miRNA nanoparticles were distributed evenly across the MAO surface. The miRNA loading increased with increasing bilayer number. More importantly, a sustained miRNA release was obtained over a timeframe of approximately 2 weeks. In vitro transfection revealed that the CS-antimiR-138 nanoparticles were taken up efficiently by the cells and caused significant knockdown of miR-138 without showing significant cytotoxicity. The CS-antimiR-138/HA PEM surface enhanced the osteogenic differentiation of MSCs in terms of enhanced alkaline phosphatase, collagen production and extracellular matrix mineralization. Substantially enhanced in vivo osseointegration was observed in the rat model.
Conclusions: The findings demonstrated that the novel CS-antimiR-138/HA PEM-functionalized microporous Ti implant exhibited sustained release of CS-antimiR-138, and notably enhanced the in vitro osteogenic differentiation of MSCs and in vivo osseointegration. This novel miRNA-functionalized Ti implant may be used in the clinical setting to allow for more effective and robust osseointegration.
Keywords: Layer-by-layer; Mesenchymal stem cells; Microarc oxidation; Sustained release; Titanium implants; microRNAs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
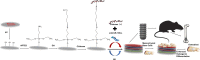







Similar articles
-
Microarc-oxidized titanium surfaces functionalized with microRNA-21-loaded chitosan/hyaluronic acid nanoparticles promote the osteogenic differentiation of human bone marrow mesenchymal stem cells.Int J Nanomedicine. 2015 Oct 27;10:6675-87. doi: 10.2147/IJN.S94689. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26604744 Free PMC article.
-
MicroRNA functionalized microporous titanium oxide surface by lyophilization with enhanced osteogenic activity.ACS Appl Mater Interfaces. 2013 Apr 10;5(7):2733-44. doi: 10.1021/am400374c. Epub 2013 Mar 21. ACS Appl Mater Interfaces. 2013. PMID: 23459382
-
Surface Functionalization with Proanthocyanidins Provides an Anti-Oxidant Defense Mechanism That Improves the Long-Term Stability and Osteogenesis of Titanium Implants.Int J Nanomedicine. 2020 Mar 10;15:1643-1659. doi: 10.2147/IJN.S231339. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32210558 Free PMC article.
-
The Effect of Antibacterial-Osteogenic Surface Modification on the Osseointegration of Titanium Implants: A Static and Dynamic Strategy.ACS Biomater Sci Eng. 2024 Jul 8;10(7):4093-4113. doi: 10.1021/acsbiomaterials.3c01756. Epub 2024 Jun 3. ACS Biomater Sci Eng. 2024. PMID: 38829538 Review.
-
Growth factor-functionalized titanium implants for enhanced bone regeneration: A review.Int J Biol Macromol. 2024 Aug;274(Pt 2):133153. doi: 10.1016/j.ijbiomac.2024.133153. Epub 2024 Jun 17. Int J Biol Macromol. 2024. PMID: 38897500 Review.
Cited by
-
Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies.Polymers (Basel). 2021 Dec 31;14(1):165. doi: 10.3390/polym14010165. Polymers (Basel). 2021. PMID: 35012187 Free PMC article. Review.
-
Functional engineering strategies of 3D printed implants for hard tissue replacement.Regen Biomater. 2022 Nov 24;10:rbac094. doi: 10.1093/rb/rbac094. eCollection 2023. Regen Biomater. 2022. PMID: 36683758 Free PMC article. Review.
-
circRNA422 enhanced osteogenic differentiation of bone marrow mesenchymal stem cells during early osseointegration through the SP7/LRP5 axis.Mol Ther. 2022 Oct 5;30(10):3226-3240. doi: 10.1016/j.ymthe.2022.05.020. Epub 2022 May 31. Mol Ther. 2022. PMID: 35642253 Free PMC article.
-
Enhanced bacteriostasis and osseointegrative properties of SiRNA-modified polyetheretherketone surface for implant applications.PLoS One. 2024 Dec 5;19(12):e0314091. doi: 10.1371/journal.pone.0314091. eCollection 2024. PLoS One. 2024. PMID: 39636795 Free PMC article.
-
Functional hydrogel empowering 3D printing titanium alloys.Mater Today Bio. 2024 Dec 24;30:101422. doi: 10.1016/j.mtbio.2024.101422. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39830135 Free PMC article. Review.
References
-
- Buser D, Sennerby L, De Bruyn H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000;2017(73):7–21. - PubMed
-
- Civantos A, Martínez-Campos E, Ramos V, Elvira C, Gallardo A, Abarrategi A. Titanium coatings and surface modifications: toward clinically useful bioactive implants. ACS Biomater Sci Eng. 2017;3:1245–1261. - PubMed
-
- Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–533. - PubMed
-
- Park JW, Jang JH, Lee CS, Hanawa T. Osteoconductivity of hydrophilic microstructured titanium implants with phosphate ion chemistry. Acta Biomater. 2009;5:2311–2321. - PubMed
-
- Iwata N, Nozaki K, Horiuchi N, Yamashita K, Tsutsumi Y, Miura H, Nagai A. Effects of controlled micro-/nanosurfaces on osteoblast proliferation. J Biomed Mater Res A. 2017;105:2589–2596. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

