Peroxiredoxin 6 mediates protective function of astrocytes in Aβ proteostasis
- PMID: 32907613
- PMCID: PMC7487614
- DOI: 10.1186/s13024-020-00401-8
Peroxiredoxin 6 mediates protective function of astrocytes in Aβ proteostasis
Abstract
Background: Disruption of β-amyloid (Aβ) homeostasis is the initial culprit in Alzheimer's disease (AD) pathogenesis. Astrocytes respond to emerging Aβ plaques by altering their phenotype and function, yet molecular mechanisms governing astrocytic response and their precise role in countering Aβ deposition remain ill-defined. Peroxiredoxin (PRDX) 6 is an enzymatic protein with independent glutathione peroxidase (Gpx) and phospholipase A2 (PLA2) activities involved in repair of oxidatively damaged cell membrane lipids and cellular signaling. In the CNS, PRDX6 is uniquely expressed by astrocytes and its exact function remains unexplored.
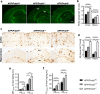
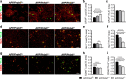
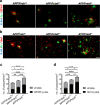
Methods: APPswe/PS1dE9 AD transgenic mice were once crossed to mice overexpressing wild-type Prdx6 allele or to Prdx6 knock out mice. Aβ pathology and associated neuritic degeneration were assessed in mice aged 10 months. Laser scanning confocal microscopy was used to characterize Aβ plaque morphology and activation of plaque-associated astrocytes and microglia. Effect of Prdx6 gene dose on plaque seeding was assessed in mice aged six months.
Results: We show that hemizygous knock in of the overexpressing Prdx6 transgene in APPswe/PS1dE9 AD transgenic mice promotes selective enticement of astrocytes to Aβ plaques and penetration of plaques by astrocytic processes along with increased number and phagocytic activation of periplaque microglia. This effects suppression of nascent plaque seeding and remodeling of mature plaques consequently curtailing brain Aβ load and Aβ-associated neuritic degeneration. Conversely, Prdx6 haplodeficiency attenuates astro- and microglia activation around Aβ plaques promoting Aβ deposition and neuritic degeneration.
Conclusions: We identify here PRDX6 as an important factor regulating response of astrocytes toward Aβ plaques. Demonstration that phagocytic activation of periplaque microglia vary directly with astrocytic PRDX6 expression level implies previously unappreciated astrocyte-guided microglia effect in Aβ proteostasis. Our showing that upregulation of PRDX6 attenuates Aβ pathology may be of therapeutic relevance for AD.
Keywords: Alzheimer’s disease; Astrocytes; Microglia; Neurodegeneration; Peroxiredoxin 6; Plaque seeding; Proteostasis; β-Amyloid plaques.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Ulland TK, Colonna M. TREM2 - a key player in microglial biology and Alzheimer disease. Nat Rev Neurol. 2018;14:667–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

