Variation in Angiostrongylus cantonensis infection in definitive and intermediate hosts in Hawaii, a global hotspot of rat lungworm disease
- PMID: 32907654
- PMCID: PMC11010199
- DOI: 10.1017/S003118202000164X
Variation in Angiostrongylus cantonensis infection in definitive and intermediate hosts in Hawaii, a global hotspot of rat lungworm disease
Abstract
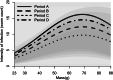
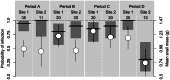
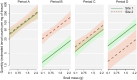
Angiostrongylus cantonensis (rat lungworm) is a tropical and subtropical parasitic nematode, with infections in humans causing angiostrongyliasis (rat lungworm disease), characterized by eosinophilic meningitis. Hawaii has been identified as a global hotspot of infection, with recent reports of high infection rates in humans, as well as rat definitive and snail intermediate hosts. This study investigated variation in A. cantonensis infection, both prevalence and intensity, in wild populations of two species of rats (Rattus exulans and R. rattus) and one species of snail (Parmarion martensi). An overall infection prevalence of 86.2% was observed in P. martensi and 63.8% in rats, with R. exulans (77.4%) greater than R. rattus (47.6%). We found infections to vary with environmental and host-related factors. Body mass was a strong predictor of infection in all three species, with different patterns seen between sexes and species of rats. Infection prevalence and intensity for R. exulans were high in May 2018 and again in February 2019, but generally lower and more variable during the intervening months. Information on sources of variability of infection in wild host populations will be a crucial component in predicting the effectiveness of future disease surveillance or targeted management strategies.
Keywords: Angiostrongyliasis; Parmarion; Rattus; drivers; eosinophilic meningitis; nematode; parasite; slug; snail; transmission.
Conflict of interest statement
None.
Figures



Similar articles
-
Insights into the biology of the rat lungworm, Angiostrongylus cantonensis.Parasit Vectors. 2025 Apr 30;18(1):163. doi: 10.1186/s13071-025-06790-3. Parasit Vectors. 2025. PMID: 40307883 Free PMC article. Review.
-
High prevalence of Angiostrongylus cantonensis (rat lungworm) on eastern Hawai'i Island: A closer look at life cycle traits and patterns of infection in wild rats (Rattus spp.).PLoS One. 2017 Dec 18;12(12):e0189458. doi: 10.1371/journal.pone.0189458. eCollection 2017. PLoS One. 2017. PMID: 29252992 Free PMC article.
-
Host snail species exhibit differential Angiostrongylus cantonensis prevalence and infection intensity across an environmental gradient.Acta Trop. 2021 Apr;216:105824. doi: 10.1016/j.actatropica.2021.105824. Epub 2021 Jan 7. Acta Trop. 2021. PMID: 33422544
-
Endemic angiostrongyliasis in the Brazilian Amazon: natural parasitism of Angiostrongylus cantonensis in Rattus rattus and R. norvegicus, and sympatric giant African land snails, Achatina fulica.Acta Trop. 2013 Jan;125(1):90-7. doi: 10.1016/j.actatropica.2012.10.001. Epub 2012 Oct 13. Acta Trop. 2013. PMID: 23072946
-
Angiostrongylus (Parastrongylus) cantonensis on intermediate and definitive hosts in Ecuador, 2014-2017.Biomedica. 2019 Jun 15;39(2):370-384. doi: 10.7705/biomedica.v39i3.4387. Biomedica. 2019. PMID: 31529823 Review. English, Spanish.
Cited by
-
Prevalence of Angiostrongylus cantonensis in definitive and intermediate hosts collected from agricultural areas in Ampayon, Butuan City, Southern Philippines.J Parasit Dis. 2023 Dec;47(4):807-814. doi: 10.1007/s12639-023-01626-2. Epub 2023 Sep 5. J Parasit Dis. 2023. PMID: 38009157 Free PMC article.
-
Angiostrongylus cantonensis and neuroangiostrongyliasis (rat lungworm disease): 2020.Parasitology. 2021 Feb;148(2):129-132. doi: 10.1017/S003118202000236X. Epub 2020 Dec 14. Parasitology. 2021. PMID: 33315004 Free PMC article. No abstract available.
-
Snail coprophagy: the encounter filter, food preferences, and rat lungworm (Angiostrongylus cantonensis) prevalence.Parasite. 2024;31:76. doi: 10.1051/parasite/2024075. Epub 2024 Dec 23. Parasite. 2024. PMID: 39715443 Free PMC article.
-
Older urban rats are infected with the zoonotic nematode Angiostrongylus cantonensis.Curr Res Parasitol Vector Borne Dis. 2024 May 21;5:100179. doi: 10.1016/j.crpvbd.2024.100179. eCollection 2024. Curr Res Parasitol Vector Borne Dis. 2024. PMID: 38845789 Free PMC article.
-
A systematic review of the diversity and virulence correlates of metastrongyle lungworms in marine mammals.Parasitology. 2023 Nov;150(13):1178-1191. doi: 10.1017/S0031182023001014. Epub 2023 Oct 20. Parasitology. 2023. PMID: 37859401 Free PMC article.
References
-
- Alicata JE (1991) The discovery of Angiostrongylus cantonensis as a cause of human eosinophilic meningitis. Parasitology Today 7, 151–153. - PubMed
-
- Anderson DR (2008) Model Based Inference in the Life Sciences: a Primer on Evidence. New York, New York, USA: Springer Science + Business Media, LLC.
-
- Asato R, Taira K, Nakamura M, Kudaka J, Itokazu K and Kawanaka M (2004) Changing epidemiology of Angiostrongyliasis cantonensis in Okinawa Prefecture, Japan. Japanese Journal of Infectious Diseases 57, 184–186. - PubMed
-
- Au ACS and Ko RC (1979) Changes in worm burden, haematological and serological response in rats after single and multiple Angiostrongylus cantonensis infections. Parasitology Research 58, 233–242. - PubMed
-
- Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, Marriott D, Harkness J, Ellis J and Stark D (2016) Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143, 1087–1118. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

