Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16:0 mediates psychological stress-induced fibromyalgia-like pain
- PMID: 32907805
- PMCID: PMC7677496
- DOI: 10.1136/annrheumdis-2020-218329
Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16:0 mediates psychological stress-induced fibromyalgia-like pain
Abstract
Objectives: Fibromyalgia is commonly considered a stress-related chronic pain disorder, and daily stressors are known triggers. However, the relation between stress and pain development remains poorly defined by clinical approaches. Also, the aetiology remains largely unknown.
Methods: We used a newly developed mouse model and lipidomic approaches to probe the causation and explore the biological plausibility for how perceived stress translates into chronic non-inflammatory pain. Clinical and lipidomic investigations of fibromyalgia were conducted for human validation.
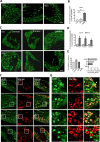
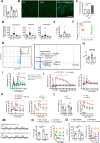
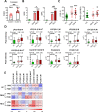
Results: Using non-painful sound stimuli as psychological stressors, we demonstrated that mice developed long-lasting non-inflammatory hyperalgesia after repeated and intermittent sound stress exposure. Elevated serum malondialdehyde level in stressed mice indicated excessive oxidative stress and lipid oxidative damage. Lipidomics revealed upregulation of lysophosphatidylcholine 16:0 (LPC16:0), a product of lipid oxidisation, in stressed mice. Intramuscular LPC16:0 injection triggered nociceptive responses and a hyperalgesic priming-like effect that caused long-lasting hypersensitivity. Pharmacological or genetic inhibition of acid-sensing ion channel 3 impeded the development of LPC16:0-induced chronic hyperalgesia. Darapladib and antioxidants could effectively alleviate the stress-induced hyperalgesia by inhibiting LPC16:0 synthesis. Clinical investigations showed that excessive oxidative stress and LPC16:0 expression also exist in patients with fibromyalgia. Moreover, LPC16:0 expression was correlated with pain symptoms in patients with high oxidative stress and disease severity.
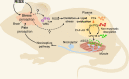
Conclusions: Our study provides experimental evidence for the causal effect of psychological stressors on chronic pain development. The findings identify a possible pathophysiological mechanism of stress-induced chronic non-inflammatory pain at molecular, behavioural and clinical levels that might indicate a new therapeutic approach for fibromyalgia.
Keywords: ASIC3; fibromyalgia; lysophosphatidylcholine; oxidative stress; pain.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

