IgE-Mediated Immune Response and Antibody-Mediated Rejection
- PMID: 32907809
- PMCID: PMC7536744
- DOI: 10.2215/CJN.02870320
IgE-Mediated Immune Response and Antibody-Mediated Rejection
Abstract
Background and objectives: Active antibody-mediated rejection is the main cause of kidney transplant loss, sharing with SLE the alloimmune response and the systemic activation of the IFN-α pathway. IgE-mediated immune response plays a key role in the development of SLE nephritis and is associated with IFN-α secretion. The aim of our study was to investigate IgE-mediated immune response in antibody-mediated rejection.
Design, setting, participants, & measurements: This was a cross-sectional study of 56 biopsy-proven antibody-mediated rejection study participants, 80 recipients with normal graft function/histology (control), 16 study participants with interstitial fibrosis/tubular atrophy, and six participants with SLE. We evaluated graft IgE deposition, tryptase (a mast cell marker), and CD203 (a specific marker of activated basophils) by immunofluorescence/confocal microscopy. In addition, we measured serum concentration of human myxovirus resistance protein 1, an IFN-α-induced protein, and anti-HLA IgE.
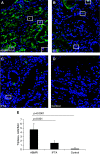
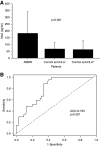
Results: We observed a significantly higher IgE deposition in tubules and glomeruli in antibody-mediated rejection (1766±79 pixels) and SLE (1495±43 pixels) compared with interstitial fibrosis/tubular atrophy (582±122 pixels) and control (253±50 pixels). Patients with antibody-mediated rejection, but not control patients and patients with interstitial fibrosis/tubular atrophy, presented circulating anti-HLA IgE antibodies, although with a low mean fluorescence intensity. In addition, immunofluorescence revealed the presence of both mast cells and activated basophils in antibody-mediated rejection but not in control and interstitial fibrosis/tubular atrophy. The concentration of circulating basophils was significantly higher in antibody-mediated rejection compared with control and interstitial fibrosis/tubular atrophy. MxA serum levels were significantly higher in antibody-mediated rejection compared with control and correlated with the extent of IgE deposition.
Conclusions: Our data suggest that IgE deposition and the subsequent recruitment of basophils and mast cells within the kidney transplant might play a role in antibody-mediated rejection.
Keywords: IgE; basophils; chronic rejection; interferon alpha; mast cells.
Copyright © 2020 by the American Society of Nephrology.
Figures



Comment in
-
IgE in Antibody-Mediated Rejection: A Novel Pathogenic Mechanism?Clin J Am Soc Nephrol. 2020 Oct 7;15(10):1392-1393. doi: 10.2215/CJN.13000820. Epub 2020 Sep 9. Clin J Am Soc Nephrol. 2020. PMID: 33028604 Free PMC article. No abstract available.
References
-
- Ettinger R, Karnell JL, Henault J, Panda SK, Riggs JM, Kolbeck R, Sanjuan MA: Pathogenic mechanisms of IgE-mediated inflammation in self-destructive autoimmune responses. Autoimmunity 50: 25–36, 2017. - PubMed
-
- Rönnblom L, Elkon KB: Cytokines as therapeutic targets in SLE. Nat Rev Rheumatol 6: 339–347, 2010. - PubMed
-
- Atta AM, Sousa CP, Carvalho EM, Sousa-Atta ML: Immunoglobulin E and systemic lupus erythematosus. Braz J Med Biol Res 37: 1497–1501, 2004. - PubMed
-
- Atta AM, Santiago MB, Guerra FG, Pereira MM, Sousa Atta ML: Autoimmune response of IgE antibodies to cellular self-antigens in systemic lupus erythematosus. Int Arch Allergy Immunol 152: 401–406, 2010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

