TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms
- PMID: 32907830
- PMCID: PMC8223629
- DOI: 10.1136/gutjnl-2019-319227
TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms
Abstract
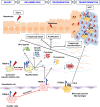
Objective: Hepatocellular carcinoma (HCC) is a prevalent and aggressive cancer usually arising on a background of chronic liver injury involving inflammatory and hepatic regenerative processes. The triggering receptor expressed on myeloid cells 2 (TREM-2) is predominantly expressed in hepatic non-parenchymal cells and inhibits Toll-like receptor signalling, protecting the liver from various hepatotoxic injuries, yet its role in liver cancer is poorly defined. Here, we investigated the impact of TREM-2 on liver regeneration and hepatocarcinogenesis.
Design: TREM-2 expression was analysed in liver tissues of two independent cohorts of patients with HCC and compared with control liver samples. Experimental HCC and liver regeneration models in wild type and Trem-2-/- mice, and in vitro studies with hepatic stellate cells (HSCs) and HCC spheroids were conducted.
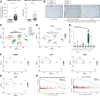
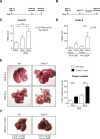
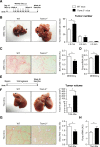
Results: TREM-2 expression was upregulated in human HCC tissue, in mouse models of liver regeneration and HCC. Trem-2-/- mice developed more liver tumours irrespective of size after diethylnitrosamine (DEN) administration, displayed exacerbated liver damage, inflammation, oxidative stress and hepatocyte proliferation. Administering an antioxidant diet blocked DEN-induced hepatocarcinogenesis in both genotypes. Similarly, Trem-2-/- animals developed more and larger tumours in fibrosis-associated HCC models. Trem-2-/- livers showed increased hepatocyte proliferation and inflammation after partial hepatectomy. Conditioned media from human HSCs overexpressing TREM-2 inhibited human HCC spheroid growth in vitro through attenuated Wnt ligand secretion.
Conclusion: TREM-2 plays a protective role in hepatocarcinogenesis via different pleiotropic effects, suggesting that TREM-2 agonism should be investigated as it might beneficially impact HCC pathogenesis in a multifactorial manner.
Keywords: hepatocellular carcinoma; immune-mediated liver damage; liver immunology; liver regeneration; molecular carcinogenesis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





Comment in
-
Keeping the liver fit with TREM2 during hepatic carcinogenesis.Gut. 2021 Jul;70(7):1210-1211. doi: 10.1136/gutjnl-2020-322638. Epub 2020 Nov 4. Gut. 2021. PMID: 33148554 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
