Rationale and design of a randomised, double-blind, placebo-controlled, parallel-group, investigator-initiated phase 2a study to investigate the efficacy and safety of elobixibat in combination with cholestyramine for non-alcoholic fatty liver disease
- PMID: 32907904
- PMCID: PMC7482497
- DOI: 10.1136/bmjopen-2020-037961
Rationale and design of a randomised, double-blind, placebo-controlled, parallel-group, investigator-initiated phase 2a study to investigate the efficacy and safety of elobixibat in combination with cholestyramine for non-alcoholic fatty liver disease
Abstract
Introduction: Non-alcoholic fatty liver disease (NAFLD) pathogenesis involves abnormal metabolism of cholesterol and hepatic accumulation of toxic free-cholesterol. Elobixibat (EXB) inhibits the ileal bile acid (BA) transporter. EXB and cholestyramine (CTM) facilitate the removal of free cholesterol from the liver by decreasing BA recirculation to the liver, thereby stimulating novel BA synthesis from cholesterol. In this randomised, double-blind, placebo-controlled, parallel-group, phase IIa study, we aim to provide a proof-of-concept assessment by evaluating the efficacy and safety of EXB in combination with CTM in patients with NAFLD.
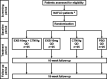
Methods and analysis: A total of 100 adult patients with NAFLD, diagnosed based on low-density lipoprotein cholesterol (LDL-C) level of >120 mg/dL and liver fat content of ≥8% by MRI-based proton density fat fraction (MRI-PDFF), who meet the inclusion/exclusion criteria will be enrolled. The patients will be randomly assigned to receive the combination therapy of 10 mg EXB and 9 g CTM powder (4 g CTM), 10 mg EXB monotherapy, 9 g CTM powder monotherapy or a placebo treatment (n=25 per group). Blood tests and MRIs will be performed 16 weeks following treatment initiation. The primary study endpoint will be the absolute LDL-C level change at week 16 after treatment initiation. The exploratory endpoint will include absolute changes in the liver fat fraction as measured by MRI-PDFF. This proof-of-concept study will determine whether the combination therapy of EXB and CTM is effective and safe for patients with NAFLD.
Ethics and dissemination: Ethics approval was obtained from the Ethics Committee of Yokohama City University Hospital before participant enrolment. The results of this study will be submitted for publication in international peer-reviewed journals and the key findings will be presented at international scientific conferences.
Trial registration number: NCT04235205.
Keywords: adult gastroenterology; clinical trials; gastroenterology; hepatobiliary disease.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Data will be retained in accordance with the Japanese ethical guidelines for clinical research. Participants will be allocated a unique identification (ID) number at entry. The master list linking participant personal information and ID number will be maintained in a separate locked cabinet and password-protected hard drive. Data will be analysed by ID number only. Records will be retained for 5 years after study completion, and then destroyed by the data center. AN reports grants and research support from Gilead, Mylan EPD, EA Pharma, Kowa, Taisho, Biofermin; is a consulting adviser for Gilead, Boehringer Ingelheim, BMS, Kowa, Astellas, EA Pharma, Mylan EPD. Other authors declare no competing interests.
Figures
Similar articles
-
Rationale and design of a multicentre, 12-week, randomised, double-blind, placebo-controlled, parallel-group, investigator-initiated trial to investigate the efficacy and safety of elobixibat for chronic constipation.BMJ Open. 2022 May 30;12(5):e060704. doi: 10.1136/bmjopen-2021-060704. BMJ Open. 2022. PMID: 35636802 Free PMC article. Clinical Trial.
-
Efficacy and safety of guanabenz acetate treatment for non-alcoholic fatty liver disease: a study protocol for a randomised investigator-initiated phase IIa study.BMJ Open. 2022 Jul 12;12(7):e060335. doi: 10.1136/bmjopen-2021-060335. BMJ Open. 2022. PMID: 35820743 Free PMC article.
-
Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study.Diabetologia. 2018 Sep;61(9):1923-1934. doi: 10.1007/s00125-018-4675-2. Epub 2018 Jul 3. Diabetologia. 2018. PMID: 29971527 Free PMC article. Clinical Trial.
-
Efficacy and safety of PXL770, a direct AMP kinase activator, for the treatment of non-alcoholic fatty liver disease (STAMP-NAFLD): a randomised, double-blind, placebo-controlled, phase 2a study.Lancet Gastroenterol Hepatol. 2021 Nov;6(11):889-902. doi: 10.1016/S2468-1253(21)00300-9. Epub 2021 Sep 22. Lancet Gastroenterol Hepatol. 2021. PMID: 34560015 Clinical Trial.
-
Fluvastatin in combination with other lipid-lowering agents.Br J Clin Pract Suppl. 1996 Jan;77A:28-32. Br J Clin Pract Suppl. 1996. PMID: 8729588 Review.
Cited by
-
Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications.Front Cell Infect Microbiol. 2022 Nov 8;12:997018. doi: 10.3389/fcimb.2022.997018. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36425787 Free PMC article. Review.
-
Is there a role of lipid-lowering therapies in the management of fatty liver disease?World J Hepatol. 2022 Jan 27;14(1):119-139. doi: 10.4254/wjh.v14.i1.119. World J Hepatol. 2022. PMID: 35126843 Free PMC article. Review.
-
Combined Use of Bicyclol and Berberine Alleviates Mouse Nonalcoholic Fatty Liver Disease.Front Pharmacol. 2022 Feb 16;13:843872. doi: 10.3389/fphar.2022.843872. eCollection 2022. Front Pharmacol. 2022. PMID: 35250593 Free PMC article.
-
Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases.Acta Pharm Sin B. 2022 May;12(5):2129-2149. doi: 10.1016/j.apsb.2021.12.011. Epub 2021 Dec 22. Acta Pharm Sin B. 2022. PMID: 35646540 Free PMC article. Review.
-
Efficacy and underlying mechanisms of berberine against lipid metabolic diseases: a review.Front Pharmacol. 2023 Nov 15;14:1283784. doi: 10.3389/fphar.2023.1283784. eCollection 2023. Front Pharmacol. 2023. PMID: 38034996 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical