Molecular regulation of ZmMs7 required for maize male fertility and development of a dominant male-sterility system in multiple species
- PMID: 32907946
- PMCID: PMC7519223
- DOI: 10.1073/pnas.2010255117
Molecular regulation of ZmMs7 required for maize male fertility and development of a dominant male-sterility system in multiple species
Abstract
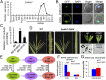
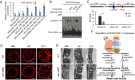
Understanding the molecular basis of male sterility and developing practical male-sterility systems are essential for heterosis utilization and commercial hybrid seed production in crops. Here, we report molecular regulation by genic male-sterility gene maize male sterility 7 (ZmMs7) and its application for developing a dominant male-sterility system in multiple species. ZmMs7 is specifically expressed in maize anthers, encodes a plant homeodomain (PHD) finger protein that functions as a transcriptional activator, and plays a key role in tapetal development and pollen exine formation. ZmMs7 can interact with maize nuclear factor Y (NF-Y) subunits to form ZmMs7-NF-YA6-YB2-YC9/12/15 protein complexes that activate target genes by directly binding to CCAAT box in their promoter regions. Premature expression of ZmMs7 in maize by an anther-specific promoter p5126 results in dominant and complete male sterility but normal vegetative growth and female fertility. Early expression of ZmMs7 downstream genes induced by prematurely expressed ZmMs7 leads to abnormal tapetal development and pollen exine formation in p5126-ZmMs7 maize lines. The p5126-ZmMs7 transgenic rice and Arabidopsis plants display similar dominant male sterility. Meanwhile, the mCherry gene coupled with p5126-ZmMs7 facilitates the sorting of dominant sterility seeds based on fluorescent selection. In addition, both the ms7-6007 recessive male-sterility line and p5126-ZmMs7M dominant male-sterility line are highly stable under different genetic germplasms and thus applicable for hybrid maize breeding. Together, our work provides insight into the mechanisms of anther and pollen development and a promising technology for hybrid seed production in crops.
Keywords: PHD finger; ZmMs7; dominant male-sterility system; protein-protein interaction.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Tester M., Langridge P., Breeding technologies to increase crop production in a changing world. Science 327, 818–822 (2010). - PubMed
-
- Wan X. et al. ., Maize genic male-sterility genes and their applications in hybrid breeding: Progress and perspectives. Mol. Plant 12, 321–342 (2019). - PubMed
-
- Chen L., Liu Y.-G., Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 65, 579–606 (2014). - PubMed
-
- Kim Y.-J., Zhang D., Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci. 23, 53–65 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

