Binding of Leishmania infantum Lipophosphoglycan to the Midgut Is Not Sufficient To Define Vector Competence in Lutzomyia longipalpis Sand Flies
- PMID: 32907950
- PMCID: PMC7485685
- DOI: 10.1128/mSphere.00594-20
Binding of Leishmania infantum Lipophosphoglycan to the Midgut Is Not Sufficient To Define Vector Competence in Lutzomyia longipalpis Sand Flies
Abstract
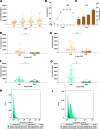
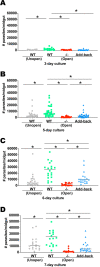
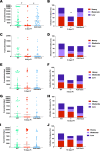
The major surface lipophosphoglycan (LPG) of Leishmania parasites is critical to vector competence in restrictive sand fly vectors in mediating Leishmania attachment to the midgut epithelium, considered essential to parasite survival and development. However, the relevance of LPG for sand flies that harbor multiple species of Leishmania remains elusive. We tested binding of Leishmania infantum wild-type (WT), LPG-defective (Δlpg1 mutants), and add-back (Δlpg1 + LPG1) lines to sand fly midguts in vitro and their survival in Lutzomyia longipalpis sand flies in vivoLe. infantum WT parasites attached to the Lu. longipalpis midgut in vitro, with late-stage parasites binding to midguts in significantly higher numbers than were seen with early-stage promastigotes. Δlpg1 mutants did not bind to Lu. longipalpis midguts, and this was rescued in the Δlpg1 + LPG1 lines, indicating that midgut binding is mediated by LPG. When Lu. longipalpis sand flies were infected with the Le. infantum WT or Le. infantum Δlpg1 or Le. infantum Δlpg1 + LPG1 line of the BH46 or BA262 strains, the BH46 Δlpg1 mutant, but not the BA262 Δlpg1 mutant, survived and grew to numbers similar to those seen with the WT and Δlpg1 + LPG1 lines. Exposure of BH46 and BA262 Δlpg1 mutants to blood-engorged midgut extracts led to mortality of the BA262 Δlpg1 but not the BH46 Δlpg1 parasites. These findings suggest that Le. infantum LPG protects parasites on a strain-specific basis early in infection, likely against toxic components of blood digestion, but that it is not necessary to prevent Le. infantum evacuation along with the feces in the permissive vector Lu. longipalpisIMPORTANCE It is well established that the presence of LPG is sufficient to define the vector competence of restrictive sand fly vectors with respect to Leishmania parasites. However, the permissiveness of other sand flies with respect to multiple Leishmania species suggests that other factors might define vector competence for these vectors. In this study, we investigated the underpinnings of Leishmania infantum survival and development in its natural vector, Lutzomyia longipalpis We found that LPG-mediated midgut binding persists in late-stage parasites. This observation is of relevance for the understanding of vector-parasite molecular interactions and suggests that only a subset of infective metacyclic-stage parasites (metacyclics) lose their ability to attach to the midgut, with implications for parasite transmission dynamics. However, our data also demonstrate that LPG is not a determining factor in Leishmania infantum retention in the midgut of Lutzomyia longipalpis, a permissive vector. Rather, LPG appears to be more important in protecting some parasite strains from the toxic environment generated during blood meal digestion in the insect gut. Thus, the relevance of LPG in parasite development in permissive vectors appears to be a complex issue and should be investigated on a strain-specific basis.
Keywords: LPG; Leishmania; parasite binding; parasite survival; sand fly; sand fly midgut; vector competence.
Figures




Similar articles
-
Pathogen-associated molecular patterns (PAMPs) derived from Leishmania and bacteria increase gene expression of antimicrobial peptides and gut surface proteins in sand flies.Int J Parasitol. 2024 Aug;54(10):485-495. doi: 10.1016/j.ijpara.2024.04.005. Epub 2024 Apr 16. Int J Parasitol. 2024. PMID: 38626865
-
Leishmania chagasi: lipophosphoglycan characterization and binding to the midgut of the sand fly vector Lutzomyia longipalpis.Mol Biochem Parasitol. 2002 May;121(2):213-24. doi: 10.1016/s0166-6851(02)00033-6. Mol Biochem Parasitol. 2002. PMID: 12034455
-
The Gut Microbiome of the Vector Lutzomyia longipalpis Is Essential for Survival of Leishmania infantum.mBio. 2017 Jan 17;8(1):e01121-16. doi: 10.1128/mBio.01121-16. mBio. 2017. PMID: 28096483 Free PMC article.
-
Leishmania development in sand flies: parasite-vector interactions overview.Parasit Vectors. 2012 Dec 3;5:276. doi: 10.1186/1756-3305-5-276. Parasit Vectors. 2012. PMID: 23206339 Free PMC article. Review.
-
Midgut and stomodeal valve attachment of Leishmania in sand flies.Trends Parasitol. 2025 Sep;41(9):769-779. doi: 10.1016/j.pt.2025.06.017. Epub 2025 Jul 22. Trends Parasitol. 2025. PMID: 40701917 Review.
Cited by
-
Leishmania-sand fly interactions: exploring the role of the immune response and potential strategies for Leishmaniasis control.J Parasit Dis. 2024 Dec;48(4):655-670. doi: 10.1007/s12639-024-01684-0. Epub 2024 May 13. J Parasit Dis. 2024. PMID: 39493480 Review.
-
In vitro interaction profiles and midgut glycoconjugates of Trichophoromyia spp./Leishmania (Viannia) lainsoni.Parasit Vectors. 2025 Jun 9;18(1):216. doi: 10.1186/s13071-025-06860-6. Parasit Vectors. 2025. PMID: 40490757 Free PMC article.
-
Phenotypical Differences between Leishmania (Leishmania) amazonensis PH8 and LV79 Strains May Impact Survival in Mammal Host and in Phlebotomine Sand Flies.Pathogens. 2023 Jan 22;12(2):173. doi: 10.3390/pathogens12020173. Pathogens. 2023. PMID: 36839445 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
