Subcellular Localization of MxB Determines Its Antiviral Potential against Influenza A Virus
- PMID: 32907985
- PMCID: PMC7592211
- DOI: 10.1128/JVI.00125-20
Subcellular Localization of MxB Determines Its Antiviral Potential against Influenza A Virus
Abstract
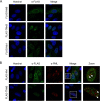
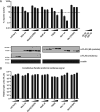
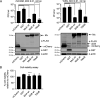
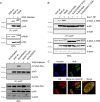
Mx proteins are interferon (IFN) type I (α/β)- and type III (λ)-induced effector proteins with intrinsic antiviral activity. Mammalian Mx proteins show different subcellular localizations and distinct yet partially overlapping viral specificities. However, the precise mechanism(s) of antiviral action are still unresolved. Human MxA accumulates in the cytoplasm and inhibits a wide variety of RNA and DNA viruses, among them influenza A virus (IAV). In contrast, MxB, the second human Mx protein, localizes via its amino (N) terminus to the outer nuclear membrane at or near nuclear pores and inhibits the nuclear import of incoming human immunodeficiency viruses (HIV) and herpesviruses, but not that of IAV. Here, we evaluated whether the antiviral specificity of MxB is determined by its subcellular localization. For this purpose, we redirected MxB to the nucleus or cytoplasm by either attaching a nuclear localization signal to its N terminus or by exchanging the N terminus of MxB with that of MxA. Interestingly, ectopic expression of these MxB variants in the nucleus or in the cytoplasm rendered the host cells resistant to IAV, revealing that the capacity of MxB to block IAV replication critically depends on the site where the protein accumulates in the infected cell. Furthermore, coimmunoprecipitation (co-IP) assays demonstrated that MxB physically interacted with the nucleoprotein (NP) of IAV. Taken together, the data indicate that the subcellular localization of the MxB protein plays a pivotal role in determining its antiviral specificity.IMPORTANCE The interferon system plays a pivotal role in the defense against viral infections. The dynamin-related Mx proteins form a small family of interferon-induced effector proteins with distinct antiviral specificities and subcellular localizations. So far, it is not clear whether the different virus specificities of Mx proteins are the result of distinct mechanisms of action or are due rather to their different subcellular localization. We show here that the human MxB protein, normally localized to the outer membrane of the cell nucleus, acquires antiviral activity against IAV when redirected to the nucleus or cytoplasm, subcellular sites where other members of the Mx protein family efficiently interfere with IAV replication. Our findings thus strongly suggest that Mx proteins act primarily through a common mechanism and that their viral specificity is at least in part determined by their individual subcellular localization.
Keywords: Mx proteins; MxB; influenza A viruses; interferon system; subcellular localization; viral specificity.
Copyright © 2020 Steiner and Pavlovic.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

