Membrane-bound sn-1,2-diacylglycerols explain the dissociation of hepatic insulin resistance from hepatic steatosis in MTTP knockout mice
- PMID: 32907986
- PMCID: PMC7707176
- DOI: 10.1194/jlr.RA119000586
Membrane-bound sn-1,2-diacylglycerols explain the dissociation of hepatic insulin resistance from hepatic steatosis in MTTP knockout mice
Abstract
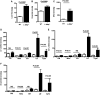
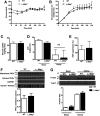
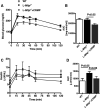
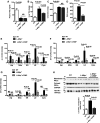
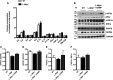
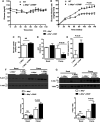
Microsomal triglyceride transfer protein (MTTP) deficiency results in a syndrome of hypolipidemia and accelerated NAFLD. Animal models of decreased hepatic MTTP activity have revealed an unexplained dissociation between hepatic steatosis and hepatic insulin resistance. Here, we performed comprehensive metabolic phenotyping of liver-specific MTTP knockout (L-Mttp-/-) mice and age-weight matched wild-type control mice. Young (10-12-week-old) L-Mttp-/- mice exhibited hepatic steatosis and increased DAG content; however, the increase in hepatic DAG content was partitioned to the lipid droplet and was not increased in the plasma membrane. Young L-Mttp-/- mice also manifested normal hepatic insulin sensitivity, as assessed by hyperinsulinemic-euglycemic clamps, no PKCε activation, and normal hepatic insulin signaling from the insulin receptor through AKT Ser/Thr kinase. In contrast, aged (10-month-old) L-Mttp-/- mice exhibited glucose intolerance and hepatic insulin resistance along with an increase in hepatic plasma membrane sn-1,2-DAG content and PKCε activation. Treatment with a functionally liver-targeted mitochondrial uncoupler protected the aged L-Mttp-/- mice against the development of hepatic steatosis, increased plasma membrane sn-1,2-DAG content, PKCε activation, and hepatic insulin resistance. Furthermore, increased hepatic insulin sensitivity in the aged controlled-release mitochondrial protonophore-treated L-Mttp-/- mice was not associated with any reductions in hepatic ceramide content. Taken together, these data demonstrate that differences in the intracellular compartmentation of sn-1,2-DAGs in the lipid droplet versus plasma membrane explains the dissociation of NAFLD/lipid-induced hepatic insulin resistance in young L-Mttp-/- mice as well as the development of lipid-induced hepatic insulin resistance in aged L-Mttp-/- mice.
Keywords: diabetes; drug therapy; lipids; liver; liver microsomal triglyceride transfer protein; liver-targeted mitochondrial uncoupler; metabolic disease; nonalcoholic fatty liver disease.
Copyright © 2020 Abulizi et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
Conflict of interest—G.I.S. is an inventor on the Yale University patent for CRMP and scientific co-founder of TLC Inc., which is developing livertargeted mitochondrial agents (including CRMP) for the treatment of NAFLD/NASH and associated metabolic diseases. There are no other conflicts of interest with the contents of this article.
Figures

References
-
- Minehira K., Young S. G., Villanueva C. J., Yetukuri L., Oresic M., Hellerstein M. K., Farese R. V. Jr., Horton J. D., Preitner F., Thorens B., et al. . 2008. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 49: 2038–2044. - PMC - PubMed
-
- Hussain M. M., Shi J., and Dreizen P.. 2003. Microsomal triglyceride transfer protein and its role in apolipoprotein B-lipoprotein assembly. J. Lipid Res. 44: 22–32. - PubMed
-
- Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., and Gregg R. E.. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258: 999–1001. - PubMed
-
- Berberich A. J., and Hegele R. A.. 2017. Lomitapide for the treatment of hypercholesterolemia. Expert Opin. Pharmacother. 18: 1261–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

