Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion
- PMID: 32908154
- PMCID: PMC7481212
- DOI: 10.1038/s41467-020-18298-8
Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion
Abstract
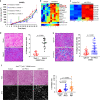
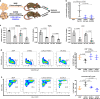
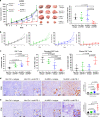
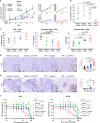
Tumor extracellular matrix has been associated with drug resistance and immune suppression. Here, proteomic and RNA profiling reveal increased collagen levels in lung tumors resistant to PD-1/PD-L1 blockade. Additionally, elevated collagen correlates with decreased total CD8+ T cells and increased exhausted CD8+ T cell subpopulations in murine and human lung tumors. Collagen-induced T cell exhaustion occurs through the receptor LAIR1, which is upregulated following CD18 interaction with collagen, and induces T cell exhaustion through SHP-1. Reduction in tumor collagen deposition through LOXL2 suppression increases T cell infiltration, diminishes exhausted T cells, and abrogates resistance to anti-PD-L1. Abrogating LAIR1 immunosuppression through LAIR2 overexpression or SHP-1 inhibition sensitizes resistant lung tumors to anti-PD-1. Clinically, increased collagen, LAIR1, and TIM-3 expression in melanoma patients treated with PD-1 blockade predict poorer survival and response. Our study identifies collagen and LAIR1 as potential markers for immunotherapy resistance and validates multiple promising therapeutic combinations.
Conflict of interest statement
D.L.G. declares advisory board/consulting work for Janssen, AstraZeneca, GlaxoSmithKline, Astellas, Ribon Therapeutics and Sanofi. D.L.G. receives research grant funding from AstraZeneca, Janssen, Ribon Therapeutics, Astellas and Takeda. L.A.B. declares consulting work for AstraZeneca, AbbVie, GenMab, BergenBio, Pharma Mar, SA. L.A.B. receives research grant funding from AbbVie, AstraZeneca, GenMab, Tolero Pharmaceuticals. All other authors declare that they have no conflict of interests.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. - PubMed
-
- Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018;15:81–94. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

