Bidirectional alterations in antibiotics susceptibility in Staphylococcus aureus-Pseudomonas aeruginosa dual-species biofilm
- PMID: 32908166
- PMCID: PMC7481796
- DOI: 10.1038/s41598-020-71834-w
Bidirectional alterations in antibiotics susceptibility in Staphylococcus aureus-Pseudomonas aeruginosa dual-species biofilm
Abstract
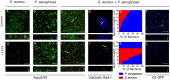
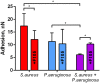
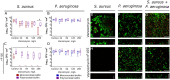
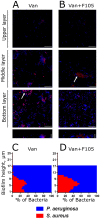
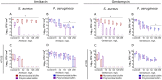
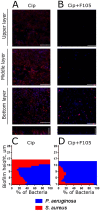
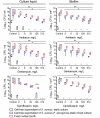
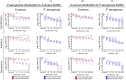
In mixed infections, the bacterial susceptibility differs significantly compared to monocultures of bacteria, and generally the concentrations of antibiotics required for the treatment increases drastically. For S. aureus and P. aeruginosa dual species biofilms, it has been numerously reported that P. aeruginosa decreases S. aureus susceptibility to a broad range of antibiotics, including beta-lactams, glycopeptides, aminoglycosides, macrolides, while sensitizes to quinolones via secretion of various metabolites. Here we show that S. aureus also modulates the susceptibility of P. aeruginosa to antibiotics in mixed cultures. Thus, S. aureus-P. aeruginosa consortium was characterized by tenfold increase in susceptibility to ciprofloxacin and aminoglycosides compared to monocultures. The same effect could be also achieved by the addition of cell-free culture of S. aureus to P. aeruginosa biofilm. Moreover, similar increase in antibiotics efficacy could be observed following addition of S. aureus suspension to the P. aeruginosa mature biofilm, compared to P. aeruginosa monoculture, and vice versa. These findings open promising perspectives to increase the antimicrobial treatment efficacy of the wounds infected with nosocomial pathogens by the transplantation of the skin residential microflora.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-Targeting Antiseptics and Antibiotics.mBio. 2019 Jul 30;10(4):e01501-19. doi: 10.1128/mBio.01501-19. mBio. 2019. PMID: 31363032 Free PMC article.
-
D-amino acids enhance the activity of antimicrobials against biofilms of clinical wound isolates of Staphylococcus aureus and Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2014 Aug;58(8):4353-61. doi: 10.1128/AAC.02468-14. Epub 2014 May 19. Antimicrob Agents Chemother. 2014. PMID: 24841260 Free PMC article.
-
Unravelling the potential of natural chelating agents in the control of Staphylococcus aureus and Pseudomonas aeruginosa biofilms.Eur J Med Chem. 2025 Feb 5;283:117163. doi: 10.1016/j.ejmech.2024.117163. Epub 2024 Dec 15. Eur J Med Chem. 2025. PMID: 39700872
-
Pseudomonas aeruginosa and Staphylococcus aureus communication in biofilm infections: insights through network and database construction.Crit Rev Microbiol. 2019 Sep-Nov;45(5-6):712-728. doi: 10.1080/1040841X.2019.1700209. Epub 2019 Dec 13. Crit Rev Microbiol. 2019. PMID: 31835971 Review.
-
Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms.Molecules. 2020 Oct 29;25(21):5024. doi: 10.3390/molecules25215024. Molecules. 2020. PMID: 33138250 Free PMC article. Review.
Cited by
-
NaCl-induced modulation of species distribution in a mixed P. aeruginosa / S. aureus /B.cepacia biofilm.Biofilm. 2023 Sep 4;6:100153. doi: 10.1016/j.bioflm.2023.100153. eCollection 2023 Dec 15. Biofilm. 2023. PMID: 37711514 Free PMC article.
-
Improving the Efficacy of Antimicrobials against Biofilm-Embedded Bacteria Using Bovine Hyaluronidase Azoximer (Longidaza®).Pharmaceutics. 2021 Oct 20;13(11):1740. doi: 10.3390/pharmaceutics13111740. Pharmaceutics. 2021. PMID: 34834156 Free PMC article.
-
Bacteriophage-Loaded Poly(lactic-co-glycolic acid) Microparticles Mitigate Staphylococcus aureus Infection and Cocultures of Staphylococcus aureus and Pseudomonas aeruginosa.Adv Healthc Mater. 2022 May;11(10):e2102539. doi: 10.1002/adhm.202102539. Epub 2022 Jan 7. Adv Healthc Mater. 2022. PMID: 34957709 Free PMC article.
-
Mussel-Inspired Sonochemical Nanocomposite Coating on Catheters for Prevention of Urinary Infections.ACS Appl Mater Interfaces. 2024 Jul 10;16(27):34656-34668. doi: 10.1021/acsami.4c05713. Epub 2024 Jun 25. ACS Appl Mater Interfaces. 2024. PMID: 38916599 Free PMC article.
-
Ceragenin Nanogel Coating Prevents Biofilms Formation on Urinary Catheters.ACS Appl Mater Interfaces. 2025 Aug 6;17(31):44319-44332. doi: 10.1021/acsami.5c13979. Epub 2025 Jul 22. ACS Appl Mater Interfaces. 2025. PMID: 40694658 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

