Stomach microbiota, Helicobacter pylori, and group 2 innate lymphoid cells
- PMID: 32908209
- PMCID: PMC8080604
- DOI: 10.1038/s12276-020-00485-8
Stomach microbiota, Helicobacter pylori, and group 2 innate lymphoid cells
Abstract
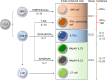
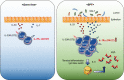
The stomach has been thought to host few commensal bacteria because of the existence of barriers, such as gastric acid. However, recent culture-independent, sequencing-based microbial analysis has shown that the stomach also harbors a wide diversity of microbiota. Although the stomach immune system, especially innate lymphoid cells (ILCs), has not been well elucidated, recent studies have shown that group 2 ILCs (ILC2s) are the dominant subtype in the stomach of both humans and mice. Stomach ILC2s are unique in that their existence is dependent on stomach microbiota, in sharp contrast to the lack of an impact of commensal microbiota on ILC2s in other tissues. The microbiota dependency of stomach ILC2s is partly explained by their responsiveness to interleukin (IL)-7. Stomach ILC2s express significantly higher IL-7 receptor protein levels on their surface and proliferate more in response to IL-7 stimulation in vitro than small intestinal ILC2s. Consistently, the stomach expresses much higher IL-7 protein levels than the small intestine. IL-5 secreted from stomach ILC2s promotes immunoglobulin (Ig) A production by plasma B cells. In a murine model, stomach ILC2s are important in containing Helicobacter pylori infection, especially in the early phase of infection, by promoting IgA production.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Bacteria-Induced Group 2 Innate Lymphoid Cells in the Stomach Provide Immune Protection through Induction of IgA.Immunity. 2020 Apr 14;52(4):635-649.e4. doi: 10.1016/j.immuni.2020.03.002. Epub 2020 Apr 1. Immunity. 2020. PMID: 32240600
-
Gastric Helicobacter pylori Infection Affects Local and Distant Microbial Populations and Host Responses.Cell Rep. 2016 Feb 16;14(6):1395-1407. doi: 10.1016/j.celrep.2016.01.017. Epub 2016 Feb 4. Cell Rep. 2016. PMID: 26854236 Free PMC article.
-
New perspectives on gastric disorders: the relationship between innate lymphoid cells and microbes in the stomach.Cell Mol Life Sci. 2025 Mar 13;82(1):113. doi: 10.1007/s00018-025-05632-w. Cell Mol Life Sci. 2025. PMID: 40074935 Free PMC article. Review.
-
The Effects of Helicobacter pylori Infection on Microbiota Associated With Gastric Mucosa and Immune Factors in Children.Front Immunol. 2021 Mar 24;12:625586. doi: 10.3389/fimmu.2021.625586. eCollection 2021. Front Immunol. 2021. PMID: 33841407 Free PMC article.
-
Dysbiotic infection in the stomach.World J Gastroenterol. 2015 Oct 28;21(40):11450-7. doi: 10.3748/wjg.v21.i40.11450. World J Gastroenterol. 2015. PMID: 26523109 Free PMC article. Review.
Cited by
-
The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis.Front Cell Infect Microbiol. 2022 Aug 15;12:953718. doi: 10.3389/fcimb.2022.953718. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36046747 Free PMC article. Review.
-
Interactions between H. pylori and the Gastric Microbiome: Impact on Gastric Homeostasis and Disease.Curr Opin Physiol. 2021 Jun;21:57-64. doi: 10.1016/j.cophys.2021.04.003. Epub 2021 Apr 24. Curr Opin Physiol. 2021. PMID: 34113748 Free PMC article.
-
Gastric microbiota: an emerging player in gastric cancer.Front Microbiol. 2023 Apr 27;14:1130001. doi: 10.3389/fmicb.2023.1130001. eCollection 2023. Front Microbiol. 2023. PMID: 37180252 Free PMC article. Review.
-
Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment.Nutrients. 2025 Jul 14;17(14):2316. doi: 10.3390/nu17142316. Nutrients. 2025. PMID: 40732941 Free PMC article. Review.
-
The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management.Gut Microbes. 2022 Jan-Dec;14(1):2108655. doi: 10.1080/19490976.2022.2108655. Gut Microbes. 2022. PMID: 35951774 Free PMC article. Review.
References
-
- Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. - PubMed
-
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977;31:107–133. - PubMed
-
- Yang I, Nell S, Suerbaum S. Survival in hostile territory: the microbiota of the stomach. FEMS Microbiol. Rev. 2013;37:736–761. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

