Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies
- PMID: 32908214
- PMCID: PMC12103240
- DOI: 10.1038/s41564-020-00789-5
Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies
Abstract
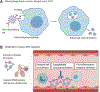
Antibody-based drugs and vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being expedited through preclinical and clinical development. Data from the study of SARS-CoV and other respiratory viruses suggest that anti-SARS-CoV-2 antibodies could exacerbate COVID-19 through antibody-dependent enhancement (ADE). Previous respiratory syncytial virus and dengue virus vaccine studies revealed human clinical safety risks related to ADE, resulting in failed vaccine trials. Here, we describe key ADE mechanisms and discuss mitigation strategies for SARS-CoV-2 vaccines and therapies in development. We also outline recently published data to evaluate the risks and opportunities for antibody-based protection against SARS-CoV-2.
Figures
References
-
- Lam TT et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

