The evolving metabolic landscape of chromatin biology and epigenetics
- PMID: 32908249
- PMCID: PMC8059378
- DOI: 10.1038/s41576-020-0270-8
The evolving metabolic landscape of chromatin biology and epigenetics
Erratum in
-
Author Correction: The evolving metabolic landscape of chromatin biology and epigenetics.Nat Rev Genet. 2020 Dec;21(12):782. doi: 10.1038/s41576-020-00291-y. Nat Rev Genet. 2020. PMID: 32978605
Abstract
Molecular inputs to chromatin via cellular metabolism are modifiers of the epigenome. These inputs - which include both nutrient availability as a result of diet and growth factor signalling - are implicated in linking the environment to the maintenance of cellular homeostasis and cell identity. Recent studies have demonstrated that these inputs are much broader than had previously been known, encompassing metabolism from a wide variety of sources, including alcohol and microbiotal metabolism. These factors modify DNA and histones and exert specific effects on cell biology, systemic physiology and pathology. In this Review, we discuss the nature of these molecular networks, highlight their role in mediating cellular responses and explore their modifiability through dietary and pharmacological interventions.
Conflict of interest statement
Competing interests
J.W.L. advises Restoration Foodworks, Nanocare Technologies and Raphael Pharmaceuticals. Z.D. and V.R. declare no competing interests.
Figures
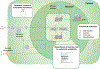


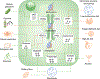
References
-
- Alberts B Molecular biology of the cell. Sixth edition. edn, (Garland Science, Taylor and Francis Group, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

