Dental Pulp Mesenchymal Stem Cells as a Treatment for Periodontal Disease in Older Adults
- PMID: 32908546
- PMCID: PMC7450326
- DOI: 10.1155/2020/8890873
Dental Pulp Mesenchymal Stem Cells as a Treatment for Periodontal Disease in Older Adults
Abstract
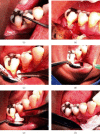
Periodontal disease (PD) is one of the main causes of tooth loss and is related to oxidative stress and chronic inflammation. Although different treatments have been proposed in the past, the vast majority do not regenerate lost tissues. In this sense, the use of dental pulp mesenchymal stem cells (DPMSCs) seems to be an alternative for the regeneration of periodontal bone tissue. A quasi-experimental study was conducted in a sample of 22 adults between 55 and 64 years of age with PD, without uncontrolled systemic chronic diseases. Two groups were formed randomly: (i) experimental group (EG) n = 11, with a treatment based on DPMSCs; and a (ii) control group (CG) n = 11, without a treatment of DPMSCs. Every participant underwent clinical and radiological evaluations and measurement of bone mineral density (BMD) by tomography. Saliva samples were taken as well, to determine the total concentration of antioxidants, superoxide dismutase (SOD), lipoperoxides, and interleukins (IL), before and 6 months after treatment. All subjects underwent curettage and periodontal surgery, the EG had a collagen scaffold treated with DPMSCs, while the CG only had the collagen scaffold placed. The EG with DPMSCs showed an increase in the BMD of the alveolar bone with a borderline statistical significance (baseline 638.82 ± 181.7 vs. posttreatment 781.26 ± 162.2 HU, p = 0.09). Regarding oxidative stress and inflammation markers, salivary SOD levels were significantly higher in EG (baseline 1.49 ± 0.96 vs. 2.14 ± 1.12 U/L posttreatment, p < 0.05) meanwhile IL1β levels had a decrease (baseline 1001.91 ± 675.5vs. posttreatment 722.3 ± 349.4 pg/ml, p < 0.05). Our findings suggest that a DPMSCs treatment based on DPMSCs has both an effect on bone regeneration linked to an increased SOD and decreased levels of IL1β in aging subjects with PD.
Copyright © 2020 Beatriz Hernández-Monjaraz et al.
Conflict of interest statement
There are no conflicts of interest.
Figures

Similar articles
-
Properties of Dental Pulp-derived Mesenchymal Stem Cells and the Effects of Culture Conditions.J Endod. 2017 Sep;43(9S):S31-S34. doi: 10.1016/j.joen.2017.06.004. Epub 2017 Aug 3. J Endod. 2017. PMID: 28781092
-
Vertical ridge augmentation using guided bone regeneration procedure and dental pulp derived mesenchymal stem cells with simultaneous dental implant placement: A histologic study in a sheep model.J Stomatol Oral Maxillofac Surg. 2019 Jun;120(3):216-223. doi: 10.1016/j.jormas.2018.12.011. Epub 2018 Dec 20. J Stomatol Oral Maxillofac Surg. 2019. PMID: 30579853
-
Guided tissue regeneration in smokers: effect of aggressive anti-infective therapy in Class II furcation defects.J Periodontol. 2003 May;74(5):579-84. doi: 10.1902/jop.2003.74.5.579. J Periodontol. 2003. PMID: 12816288 Clinical Trial.
-
Stem cell-based tooth and periodontal regeneration.Oral Dis. 2018 Jul;24(5):696-705. doi: 10.1111/odi.12703. Epub 2017 Jul 24. Oral Dis. 2018. PMID: 28636235 Review.
-
Developmental pathways of periodontal tissue regeneration: Developmental diversities of tooth morphogenesis do also map capacity of periodontal tissue regeneration?J Periodontal Res. 2019 Feb;54(1):10-26. doi: 10.1111/jre.12596. Epub 2018 Sep 12. J Periodontal Res. 2019. PMID: 30207395 Review.
Cited by
-
The Role of Dental-derived Stem Cell-based Therapy and Their Derived Extracellular Vesicles in Post-COVID-19 Syndrome-induced Tissue Damage.Stem Cell Rev Rep. 2024 Nov;20(8):2062-2103. doi: 10.1007/s12015-024-10770-y. Epub 2024 Aug 16. Stem Cell Rev Rep. 2024. PMID: 39150646 Review.
-
Impact of Oral Mesenchymal Stem Cells Applications as a Promising Therapeutic Target in the Therapy of Periodontal Disease.Int J Mol Sci. 2022 Nov 3;23(21):13419. doi: 10.3390/ijms232113419. Int J Mol Sci. 2022. PMID: 36362206 Free PMC article. Review.
-
Microarray analysis of long non-coding RNAs related to osteogenic differentiation of human dental pulp stem cells.J Dent Sci. 2022 Apr;17(2):733-743. doi: 10.1016/j.jds.2021.10.014. Epub 2021 Nov 4. J Dent Sci. 2022. PMID: 35756759 Free PMC article.
-
Human dental pulp stem cells attenuate streptozotocin-induced parotid gland injury in rats.Stem Cell Res Ther. 2021 Nov 14;12(1):577. doi: 10.1186/s13287-021-02646-6. Stem Cell Res Ther. 2021. PMID: 34775989 Free PMC article.
-
Regeneration of periodontal bone defects with mesenchymal stem cells in animal models. Systematic review and meta-analysis.Odontology. 2023 Jan;111(1):105-122. doi: 10.1007/s10266-022-00725-5. Epub 2022 Jul 5. Odontology. 2023. PMID: 35788845 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

