Protective Effects and Metabolic Regulatory Mechanisms of Shenyan Fangshuai Recipe on Chronic Kidney Disease in Rats
- PMID: 32908562
- PMCID: PMC7468650
- DOI: 10.1155/2020/5603243
Protective Effects and Metabolic Regulatory Mechanisms of Shenyan Fangshuai Recipe on Chronic Kidney Disease in Rats
Abstract
Background: Chronic kidney disease (CKD) is one of the major causes of renal damage. Shenyan Fangshuai Recipe (SFR), a modified prescription of traditional medicine in China, showed potent effects in alleviating edema, proteinuria, and hematuria of CKD in clinical practices. In this study, we aimed to investigate scientific evidence-based efficacy as well as metabolic regulations of SFR in CKD treatment.
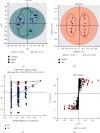
Materials and methods: The effect of SFR on CKD was observed in a rat model which is established with oral administration of adenine-ethambutol mixture for 21 days. Further, metabolites in serum were detected and identified with ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS). Metabolomics study was performed using Ingenuity Pathway Analysis (IPA) software.
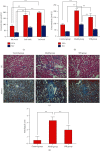
Results: With H&E staining and Masson's trichrome, the results showed that chronic kidney damage is significantly rescued with SFR treatment and recovered to an approximately normal condition. Along with 44 differential metabolites discovered, the regulation of SFR on CKD was enriched in glycine biosynthesis I, mitochondrial L-carnitine shuttle pathway, phosphatidylethanolamine biosynthesis III, sphingosine-1-phosphate signaling, L-serine degradation, folate transformations I, noradrenaline and adrenaline degradation, salvage pathways of pyrimidine ribonucleotides, cysteine biosynthesis III (Mammalia), glycine betaine degradation, and cysteine biosynthesis/homocysteine degradation. Further, TGFβ-1 and MMP-9 were observed playing roles in this regulatory process by performing immunohistochemical staining.
Conclusion: SFR exerts potent effects of alleviating glomerular sclerosis and interstitial fibrosis in the kidney, mainly via integrated regulations on metabolism and production of homocysteine, L-carnitine, and epinephrine, as well as the expression of TGFβ-1. This study provides evidence for SFR's protective effects on CKD and reveals the metabolic mechanism behind these benefits for the first time.
Copyright © 2020 Xinqi Deng et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

