Protective Role of Glutathione and Nitric Oxide Production in the Pathogenesis of Pterygium
- PMID: 32908689
- PMCID: PMC7477589
- DOI: 10.1155/2020/9638763
Protective Role of Glutathione and Nitric Oxide Production in the Pathogenesis of Pterygium
Abstract
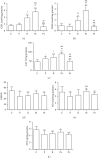
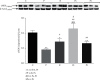
Objective: In the pathogenesis of pterygium, the protective role of glutathione and nitric oxide production is unclear. These are important factors for homeostasis in the redox state of cells. The aim of this study was to determine the levels of these and related parameters in pterygium tissue. Patients and Methods. The study sample consisted of 120 patients diagnosed with primary or recurrent pterygium. Five groups of tissue samples were examined: control, primary pterygium, recurrent pterygium, and two groups of primary pterygium given a one-month NAC presurgery treatment (topical or systemic). The levels of endothelial nitric oxide synthase (eNOS), nitric oxide (NO), 3-nitrotyrosine (3NT), reduced and oxidized glutathione (GSH and GSSG), and catalase (CAT) were evaluated in tissue homogenates.
Results: Compared with the control, decreased levels of eNOS, NO, and 3-nitrotyrosine as well as the degree of oxidation of GSH (GSSG%) were observed in primary and recurrent pterygium. 3-Nitrotyrosine and GSSG% were reduced in the other pterygium groups. GSH and CAT were enhanced in recurrent pterygium and systemic-treated primary pterygium but were unchanged for topical-treated primary pterygium. There was a strong positive correlation of eNOS with NO and 3NT, GSSG% with NO and 3NT, and GSH with GSSG and CAT. Women showed a higher level of GSH and catalase in primary pterygium, whereas a lower level of GSH and a higher level of NO in recurrent pterygium.
Conclusion: The results are congruent with the following proposed sequence of events leading to a protective response of the organism during the pathogenesis of primary pterygium: a decreased level of eNOS provokes a decline in the level of NO in pterygium tissue, which then leads to reduced S-nitrosylation of GSH or other thiols and possibly to the modulation of the intracellular level of GSH through synthesis and/or mobilization from other tissues.
Copyright © 2020 Fidelina Parra et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification.Biochemistry. 2013 Sep 24;52(38):6712-23. doi: 10.1021/bi400404s. Epub 2013 Sep 11. Biochemistry. 2013. PMID: 23977830 Free PMC article.
-
Exercise conditioning attenuates the hypertensive effects of nitric oxide synthase inhibitor in rat.Mol Cell Biochem. 2002 Feb;231(1-2):129-37. doi: 10.1023/a:1014416915643. Mol Cell Biochem. 2002. PMID: 11952154
-
Oxidant/antioxidant state in tissue of prymary and recurrent pterygium.BMC Ophthalmol. 2014 Nov 27;14:149. doi: 10.1186/1471-2415-14-149. BMC Ophthalmol. 2014. PMID: 25428713 Free PMC article.
-
Influence of sildenafil and donepezil administration on the serum redox balance in experimentally induced lower limb critical ischemia.Clujul Med. 2013;86(3):250-8. Epub 2013 Aug 5. Clujul Med. 2013. PMID: 26527957 Free PMC article. Review.
-
Glutathione in Protein Redox Modulation through S-Glutathionylation and S-Nitrosylation.Molecules. 2021 Jan 15;26(2):435. doi: 10.3390/molecules26020435. Molecules. 2021. PMID: 33467703 Free PMC article. Review.
Cited by
-
Lactobacillus sakei Pro-Bio65 Reduces TNF-α Expression and Upregulates GSH Content and Antioxidant Enzymatic Activities in Human Conjunctival Cells.Transl Vis Sci Technol. 2021 May 3;10(6):8. doi: 10.1167/tvst.10.6.8. Transl Vis Sci Technol. 2021. PMID: 34111255 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

