Analysis of Circulating Tumor DNA to Predict Neoadjuvant Therapy Effectiveness and Breast Cancer Recurrence
- PMID: 32908788
- PMCID: PMC7462818
- DOI: 10.4048/jbc.2020.23.e41
Analysis of Circulating Tumor DNA to Predict Neoadjuvant Therapy Effectiveness and Breast Cancer Recurrence
Abstract
Purpose: Real-time detection and intervention can be used as potential measures to markedly decrease breast cancer mortality. Assessment of circulating tumor DNA (ctDNA) may offer great benefits for the management of breast cancer over time. However, the use of ctDNA to predict the effectiveness of neoadjuvant treatment and recurrence of breast cancer has rarely been studied.
Methods: We prospectively recruited 31 breast cancer patients with 4 subtypes. Three time points were set in this study, including before any therapy (C1), during surgery (T), and six months after surgery (C2). We collected peripheral blood samples from all 31 patients at C1, tumor tissue from all 31 patients at T, and peripheral blood samples from 25 patients at C2. Targeted 727-gene panel sequencing was performed on ctDNA from all blood samples and tissue DNA from all tissue samples. Somatic mutations were detected and analyzed using a reference standard pipeline. Statistical analysis was performed to identify possible associations between ctDNA profiles and clinical outcomes.
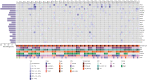
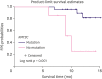
Results: In total, we detected 159, 271, and 70 somatic mutations in 30 C1 samples, 31 T samples, and 12 C2 samples, respectively. We identified specific genes, such as PIK3CA, TP53, and KMT2C, which were highly mutated in the tissue samples. Furthermore, mutated KMT2C observed in ctDNA of the C2 samples may be an indicator of breast cancer recurrence.
Conclusion: Our study highlights the potential of ctDNA analysis at different timepoints for assessing tumor progression and treatment effectiveness, as well as prediction of breast cancer recurrence.
Keywords: Breast neoplasms; Circulating tumor DNA; Neoadjuvant therapy; Recurrence.
© 2020 Korean Breast Cancer Society.
Conflict of interest statement
Conflict of Interest: The authors declare that they have no competing interests.
Figures

Similar articles
-
Dynamics of circulating tumor DNA during postoperative radiotherapy in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: a prospective observational study.Breast Cancer Res Treat. 2021 Aug;189(1):167-175. doi: 10.1007/s10549-021-06296-3. Epub 2021 Jun 21. Breast Cancer Res Treat. 2021. PMID: 34152505
-
Association of Circulating Tumor DNA and Circulating Tumor Cells After Neoadjuvant Chemotherapy With Disease Recurrence in Patients With Triple-Negative Breast Cancer: Preplanned Secondary Analysis of the BRE12-158 Randomized Clinical Trial.JAMA Oncol. 2020 Sep 1;6(9):1410-1415. doi: 10.1001/jamaoncol.2020.2295. JAMA Oncol. 2020. PMID: 32644110 Free PMC article. Clinical Trial.
-
Circulating Tumor DNA as a Predictive Marker of Recurrence for Patients With Stage II-III Breast Cancer Treated With Neoadjuvant Therapy.Front Oncol. 2021 Nov 12;11:736769. doi: 10.3389/fonc.2021.736769. eCollection 2021. Front Oncol. 2021. PMID: 34868925 Free PMC article.
-
Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: A prospective cohort study.PLoS Med. 2021 May 3;18(5):e1003620. doi: 10.1371/journal.pmed.1003620. eCollection 2021 May. PLoS Med. 2021. PMID: 33939694 Free PMC article.
-
ctDNA for Risk of Recurrence Assessment in Patients Treated with Neoadjuvant Treatment: A Systematic Review and Meta-analysis.Ann Surg Oncol. 2022 Dec;29(13):8666-8674. doi: 10.1245/s10434-022-12366-7. Epub 2022 Aug 6. Ann Surg Oncol. 2022. PMID: 35933546
Cited by
-
The modification role and tumor association with a methyltransferase: KMT2C.Front Immunol. 2024 Aug 6;15:1444923. doi: 10.3389/fimmu.2024.1444923. eCollection 2024. Front Immunol. 2024. PMID: 39165358 Free PMC article. Review.
References
-
- Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41:19–28. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

