Influence of three BALB/c substrain backgrounds on the skin tumor induction efficacy to DMBA and TPA cotreatment
- PMID: 32908818
- PMCID: PMC7469300
- DOI: 10.1186/s42826-020-00063-z
Influence of three BALB/c substrain backgrounds on the skin tumor induction efficacy to DMBA and TPA cotreatment
Erratum in
-
Correction to: Influence of three BALB/c substrain backgrounds on the skin tumor induction efficacy to DMBA and TPA cotreatment.Lab Anim Res. 2020 Oct 6;36:35. doi: 10.1186/s42826-020-00067-9. eCollection 2020. Lab Anim Res. 2020. PMID: 33042782 Free PMC article.
Abstract
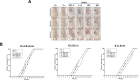
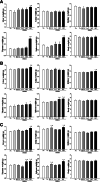
Differences in responsiveness of BALB/c substrains have been investigated in various fields, including diabetes induction, corpus callosum deficiency, virus-induced demyelinating disease, aggressive behavior and osteonecrosis. However, induction efficacy of skin tumor remains untried. We therefore investigated the influence of BALB/c substrain backgrounds on the skin tumor induction efficacy in response to DMBA (7,12-Dimethylbenz[a]anthracene) and TPA (12-O-tetradecanoylphorbol-13-acetate) cotreatment. Alterations in the levels of tumor growth related factors, histopathological structure, and the expression to tumor related proteins were measured in three BALB/c substrains (BALB/cKorl, BALB/cA and BALB/cB) after exposure to DMBA (25 μg/kg) and three different doses of TPA (2, 4 and 8 μg/kg). The average number and induction efficacy of tumors in response to DMBA+TPA treatment were significantly greater in the BALB/cKorl substrain than in BALB/cA and BALB/cB. However, cotreatment with DMBA+TPA induced similar responses for body and organ weights of all three substrains. Few differences were detected in the serum analyzing factors, while similar responsiveness was observed for blood analyzing factors after DMBA+TPA treatment. Furthermore, the three BALB/c substrains exhibited similar patterns in their histopathological structure in DMBA+TPA-induced tumors. The expression levels of apoptotic proteins and tumor related proteins were constantly maintained in all three BALB/c substrains treated with DMBA+TPA. In addition, the responsiveness to cisplatin treatment was overall very similar in the three BALB/c substrains with DMBA+TPA-induced tumors. Taken together, these results indicate that genetic background of the three BALB/c substrains does not have a major effect on the DMBA+TPA-induced skin carcinogenesis and therapeutic responsiveness of cisplatin, except induction efficacy.
Keywords: BALB/c; BALB/cKorl; Cisplatin; DMBA+TPA; Skin tumor; Substrains.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures





Similar articles
-
Correction to: Influence of three BALB/c substrain backgrounds on the skin tumor induction efficacy to DMBA and TPA cotreatment.Lab Anim Res. 2020 Oct 6;36:35. doi: 10.1186/s42826-020-00067-9. eCollection 2020. Lab Anim Res. 2020. PMID: 33042782 Free PMC article.
-
Comparison of cisplatin-induced anti-tumor response in CT26 syngeneic tumors of three BALB/c substrains.Lab Anim Res. 2021 Dec 8;37(1):33. doi: 10.1186/s42826-021-00110-3. Lab Anim Res. 2021. PMID: 34876239 Free PMC article.
-
NTP Toxicology and Carcinogenesis Studies of o-Phenylphenol (CAS No. 90-43-7) Alone and with 7,12-Dimethylbenz(a)anthracene (CAS No. 57-97-6) in Swiss CD-1 Mice (Dermal Studies).Natl Toxicol Program Tech Rep Ser. 1986 Mar;301:1-141. Natl Toxicol Program Tech Rep Ser. 1986. PMID: 12748705
-
FVB/N mice: an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin.Carcinogenesis. 1993 Nov;14(11):2353-8. doi: 10.1093/carcin/14.11.2353. Carcinogenesis. 1993. PMID: 8242866
-
SENCAR mouse skin tumorigenesis model versus other strains and stocks of mice.Environ Health Perspect. 1986 Sep;68:27-32. doi: 10.1289/ehp.866827. Environ Health Perspect. 1986. PMID: 3096709 Free PMC article. Review.
Cited by
-
The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5.Pharmaceutics. 2021 Sep 10;13(9):1439. doi: 10.3390/pharmaceutics13091439. Pharmaceutics. 2021. PMID: 34575516 Free PMC article.
-
Correction to: Influence of three BALB/c substrain backgrounds on the skin tumor induction efficacy to DMBA and TPA cotreatment.Lab Anim Res. 2020 Oct 6;36:35. doi: 10.1186/s42826-020-00067-9. eCollection 2020. Lab Anim Res. 2020. PMID: 33042782 Free PMC article.
-
The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5.Nutrients. 2021 Aug 27;13(9):2986. doi: 10.3390/nu13092986. Nutrients. 2021. PMID: 34578864 Free PMC article.
-
Comparison of cisplatin-induced anti-tumor response in CT26 syngeneic tumors of three BALB/c substrains.Lab Anim Res. 2021 Dec 8;37(1):33. doi: 10.1186/s42826-021-00110-3. Lab Anim Res. 2021. PMID: 34876239 Free PMC article.
References
-
- Potter M. History of the BALB/c family. Curr Top Microbiol Immunol. 1985;122:1–5. - PubMed
-
- Hilgers J, van Nie R, Ivanyi D, Hilkens J, Michalides R, de Moes J, Poort-Keesom R, Kroezen V, von Deimling O, Kominami R, Holmes R. Genetic differences in BALB/c sublines. Curr Top Microbiol Immunol. 1985;122:19–30. - PubMed
-
- Leiter EH, Le PH, Prochazka M, Worthen SM, Huppi K. Genetic and environmental control of diabetes induction by multi-dose streptozotocin in two BALB/c substrains. Diabetes Res. 1988;9:5–10. - PubMed
LinkOut - more resources
Full Text Sources

