A Novel Imidazopyridine Derivative Exerts Anticancer Activity by Inducing Mitochondrial Pathway-Mediated Apoptosis
- PMID: 32908894
- PMCID: PMC7468608
- DOI: 10.1155/2020/4929053
A Novel Imidazopyridine Derivative Exerts Anticancer Activity by Inducing Mitochondrial Pathway-Mediated Apoptosis
Abstract
Background: Cancer remains a major clinical challenge because of the lack of effective drug for its treatment. To find out novel cancer chemotherapeutic molecules, we explored the anticancer effect of novel imidazopyridine compound 9i and also investigated the underlying molecular mechanism.
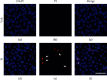
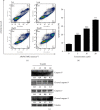
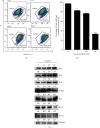
Methods: Human cervical cancer cell (HeLa) viability was measured by an MTT assay after treatment with compound 9i. Clonogenicity of HeLa cells was investigated by an in vitro colony formation assay. Cell death was visualized by propidium iodide (PI) staining. Fluorescence-activated cell sorting (FACS) was used to determine apoptosis and mitochondrial membrane potential in HeLa cells. The expression level of apoptosis-related proteins was also determined by western blot.
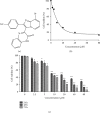
Results: Compound 9i suppressed HeLa cell viability in a time- and dose-dependent manner. Compound 9i induced mitochondrial outer membrane permeabilization (MOMP), activated caspase cascade, and finally resulted in apoptosis.
Conclusion: Compound 9i induces mitochondrial pathway-mediated apoptosis in human cervical cancer cells, suggesting that 9i could be a potential lead compound to be developed as a cancer therapeutic molecule.
Copyright © 2020 Juanli Wang et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Shinde M. H., Kshirsagar U. A. One pot synthesis of substituted imidazopyridines and thiazoles from styrenes in water assisted by NBS. Green Chemistry. 2016;18(6):1455–1458. doi: 10.1039/C5GC02771C. - DOI
-
- Martínez-Urbina M. A., Zentella A., Vilchis-Reyes M. A., et al. 6-Substituted 2-(N-trifluoroacetylamino)imidazopyridines induce cell cycle arrest and apoptosis in SK-LU-1 human cancer cell line. European Journal of Medicinal Chemistry. 2010;45(3):1211–1219. doi: 10.1016/j.ejmech.2009.11.049. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

