THAP11 Functions as a Tumor Suppressor in Gastric Cancer through Regulating c-Myc Signaling Pathways
- PMID: 32908912
- PMCID: PMC7474744
- DOI: 10.1155/2020/7838924
THAP11 Functions as a Tumor Suppressor in Gastric Cancer through Regulating c-Myc Signaling Pathways
Abstract
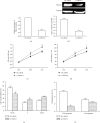
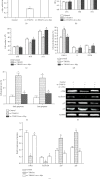
We aim to investigate the role of THAP11 (thanatos-associated protein11) in gastric cancer and its regulation mechanisms. THAP11 expression was analyzed in 51 pairs of GC tissues and the corresponding paracancerous tissues by qRT-PCR and Western blot. After THAP11 was overexpressed or knocked-down, cell proliferation, cell cycle, and apoptosis were detected in MKN-45 cells. We found that THAP11 was significantly downregulated in GC tissues and GC cell lines. Functionally, THAP11 overexpression markedly inhibited cell growth, induced G1/G0 cell-cycle arrest, and promoted cell apoptosis of MKN-45 cells, while silencing of THAP11 led to increased cell growth, increased DNA synthesis, and inhibited apoptosis. In addition, THAP11 negatively regulated the expression of c-Myc, decreased cyclinD1 protein, and increased p27 and p21 protein levels. We also found cell growth suppression induced by THAP11 was rescued by c-Myc overexpression, further confirming that THAP11 suppresses gastric cancer cell growth via the c-Myc pathway. THAP11 acts as a cell growth suppressor and exerts its role possibly through negatively regulating c-Myc pathway in gastric cancer.
Copyright © 2020 Jing Zhang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



References
-
- Balakrishnan M. P., Cilenti L., Mashak Z., Popat P., Alnemri E. S., Zervos A. S. Thap5 is a human cardiac-specific inhibitor of cell cycle that is cleaved by the proapoptotic Omi/Htra2 protease during cell death. American Journal of Physiology: Heart and Circulatory Physiology. 2009;297(2):H643–H653. doi: 10.1152/ajpheart.00234.2009. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

