Protective Effects of Thalidomide on High-Glucose-Induced Podocyte Injury through In Vitro Modulation of Macrophage M1/M2 Differentiation
- PMID: 32908940
- PMCID: PMC7474395
- DOI: 10.1155/2020/8263598
Protective Effects of Thalidomide on High-Glucose-Induced Podocyte Injury through In Vitro Modulation of Macrophage M1/M2 Differentiation
Abstract
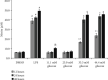
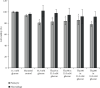
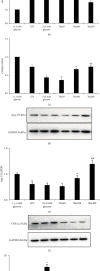
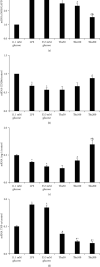
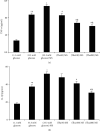
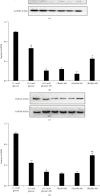
Objective. It has been shown that podocyte injury represents an important pathological basis that contributes to proteinuria and eventually leads to kidney failure. High glucose (HG) activates macrophage polarization, further exacerbating HG-induced podocyte injury. Our previous study on diabetic nephropathy rats indicated that thalidomide (Tha) has renoprotective properties. The present study explored the effects of Tha on mRNA and protein expressions of inducible nitric oxide synthase (iNOS), tumor necrosis factor- (TNF-) α, mannose receptor (CD206), and arginase- (Arg-) 1 in HG-activated macrophages. iNOS and TNF-α are established as markers of classically activated macrophage (M1). CD206 and Arg-1 are regarded as markers of alternatively activated macrophages (M2). During the experiment, the supernatants of (HG)-treated and (Tha)-treated macrophages, designated as (HG) MS and (Tha) MS, were simultaneously collected and processed. TNF-α and interleukin- (IL-) 1β levels as well as protein expressions of nephrin and podocin in HG, (HG) MS, and (Tha) MS-cultured podocytes were evaluated. The results showed that compared to the 11.1 mM normal glucose (NG), the 33.3 mM HG-cultured RAW 264.7 cells exhibited upregulated iNOS and TNF-α mRNAs and protein expressions, and downregulated CD206 and Arg-1 expressions significantly (p < 0.05). Tha 200 μg/ml suppressed iNOS and TNF-α, and promoted CD206 and Arg-1 expressions significantly compared to the HG group (p < 0.05). Furthermore, (HG) MS-treated podocytes showed an increase in TNF-α and IL-1β levels and a downregulation in nephrin and podocin expression significantly compared to NG-treated and HG-treated podocytes (p < 0.05). The (Tha 200 μg/ml) MS group exhibited a decrease in TNF-α and IL-1β level, and an upregulation in nephrin and podocin expressions significantly compared to the (HG) MS group (p < 0.05). Our research confirmed that HG-activated macrophage differentiation aggravates HG-induced podocyte injury in vitro and the protective effects of Tha might be related to its actions on TNF-α and IL-1β levels via its modulation on M1/M2 differentiation.
Copyright © 2020 Hui Liao et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Protective effects of budesonide on LPS-induced podocyte injury by modulating macrophage M1/M2 polarization: Evidence from in vitro and in silico studies.Exp Ther Med. 2022 Jul 26;24(3):589. doi: 10.3892/etm.2022.11526. eCollection 2022 Sep. Exp Ther Med. 2022. PMID: 35949344 Free PMC article.
-
Protective Effects of Two Safflower Derived Compounds, Kaempferol and Hydroxysafflor Yellow A, on Hyperglycaemic Stress-Induced Podocyte Apoptosis via Modulating of Macrophage M1/M2 Polarization.J Immunol Res. 2020 Oct 10;2020:2462039. doi: 10.1155/2020/2462039. eCollection 2020. J Immunol Res. 2020. PMID: 33102606 Free PMC article.
-
Vitamin D prevents podocyte injury via regulation of macrophage M1/M2 phenotype in diabetic nephropathy rats.Endocrinology. 2014 Dec;155(12):4939-50. doi: 10.1210/en.2014-1020. Epub 2014 Sep 4. Endocrinology. 2014. PMID: 25188527
-
[Effect of Galectin-9/Tim-3 pathway on the polarization of M1/M2 subtype in murine macrophages induced by lipopolysaccharide].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018 Sep;30(9):836-841. doi: 10.3760/cma.j.issn.2095-4352.2018.09.004. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018. PMID: 30309408 Chinese.
-
Cardiac macrophages in maintaining heart homeostasis and regulating ventricular remodeling of heart diseases.Front Immunol. 2024 Sep 20;15:1467089. doi: 10.3389/fimmu.2024.1467089. eCollection 2024. Front Immunol. 2024. PMID: 39372400 Free PMC article. Review.
Cited by
-
Protective effects of budesonide on LPS-induced podocyte injury by modulating macrophage M1/M2 polarization: Evidence from in vitro and in silico studies.Exp Ther Med. 2022 Jul 26;24(3):589. doi: 10.3892/etm.2022.11526. eCollection 2022 Sep. Exp Ther Med. 2022. PMID: 35949344 Free PMC article.
-
Targeting Macrophages: Therapeutic Approaches in Diabetic Kidney Disease.Int J Mol Sci. 2024 Apr 15;25(8):4350. doi: 10.3390/ijms25084350. Int J Mol Sci. 2024. PMID: 38673935 Free PMC article. Review.
-
Thalidomide Attenuates Colitis and Is Associated with the Suppression of M1 Macrophage Polarization by Targeting the Transcription Factor IRF5.Dig Dis Sci. 2021 Nov;66(11):3803-3812. doi: 10.1007/s10620-021-07067-2. Epub 2021 Jun 3. Dig Dis Sci. 2021. PMID: 34085173
-
RAGE-TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages.Int J Mol Sci. 2023 Apr 10;24(8):7007. doi: 10.3390/ijms24087007. Int J Mol Sci. 2023. PMID: 37108174 Free PMC article.
-
Galectin-3 Inhibition Ameliorates Streptozotocin-Induced Diabetic Cardiomyopathy in Mice.Front Cardiovasc Med. 2022 Apr 26;9:868372. doi: 10.3389/fcvm.2022.868372. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35557520 Free PMC article.
References
-
- van Beurden-Tan C. H. Y., Franken M. G., Blommestein H. M., Uyl-de Groot C. A., Sonneveld P. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. Journal of Clinical Oncology. 2017;35(12):1312–1319. doi: 10.1200/JCO.2016.71.1663. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

