Correlating Cochlear Morphometrics from Parnell's Mustached Bat (Pteronotus parnellii) with Hearing
- PMID: 32909111
- PMCID: PMC7567779
- DOI: 10.1007/s10162-020-00764-1
Correlating Cochlear Morphometrics from Parnell's Mustached Bat (Pteronotus parnellii) with Hearing
Abstract
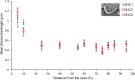
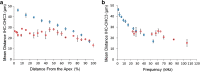
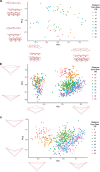
Morphometric analysis of the inner ear of mammals can provide information for cochlear frequency mapping, a species-specific designation of locations in the cochlea at which different sound frequencies are encoded. Morphometric variation occurs in the hair cells of the organ of Corti along the cochlea, with the base encoding the highest frequency sounds and the apex encoding the lowest frequencies. Changes in cell shape and spacing can yield additional information about the biophysical basis of cochlear tuning mechanisms. Here, we investigate how morphometric analysis of hair cells in mammals can be used to predict the relationship between frequency and cochlear location. We used linear and geometric morphometrics to analyze scanning electron micrographs of the hair cells of the cochleae in Parnell's mustached bat (Pteronotus parnellii) and Wistar rat (Rattus norvegicus) and determined a relationship between cochlear morphometrics and their frequency map. Sixteen of twenty-two of the morphometric parameters analyzed showed a significant change along the cochlea, including the distance between the rows of hair cells, outer hair cell width, and gap width between hair cells. A multiple linear regression model revealed that nine of these parameters are responsible for 86.9 % of the variation in these morphometric data. Determining the most biologically relevant measurements related to frequency detection can give us a greater understanding of the essential biomechanical characteristics for frequency selectivity during sound transduction in a diversity of animals.
Keywords: frequency map; geometric morphometrics; inner ear; linear morphometrics; organ of Corti.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








Similar articles
-
Mitochondrial genome of the neotropical Parnell's mustached bat Pteronotus parnellii (Pteronotus, Mormoopidae).Mitochondrial DNA. 2015;26(6):921-2. doi: 10.3109/19401736.2013.863291. Epub 2014 Jan 10. Mitochondrial DNA. 2015. PMID: 24409886
-
High-frequency two-tone distortions from the ear of the mustached bat, Pteronotus parnellii reflect enhanced cochlear tuning.Naturwissenschaften. 1992 Sep;79(9):425-7. doi: 10.1007/BF01138579. Naturwissenschaften. 1992. PMID: 1436119 No abstract available.
-
Connections and frequency representation in the auditory brainstem of the mustache bat, Pteronotus parnellii.J Comp Neurol. 1989 Dec 8;290(2):243-61. doi: 10.1002/cne.902900206. J Comp Neurol. 1989. PMID: 2592612
-
Where hearing starts: the development of the mammalian cochlea.J Anat. 2016 Feb;228(2):233-54. doi: 10.1111/joa.12314. Epub 2015 Jun 5. J Anat. 2016. PMID: 26052920 Free PMC article. Review.
-
How well do we understand the cochlea?Trends Neurosci. 1998 Apr;21(4):159-67. doi: 10.1016/s0166-2236(97)01192-2. Trends Neurosci. 1998. PMID: 9554726 Review.
Cited by
-
Selective Inner Hair Cell Loss in a Neonate Harbor Seal (Phoca vitulina).Animals (Basel). 2022 Jan 12;12(2):180. doi: 10.3390/ani12020180. Animals (Basel). 2022. PMID: 35049802 Free PMC article.
References
-
- Adams DC, Collyer ML, Kaliontzopoulou A (2018) Geomorph: software for geometric morphometric analyses. R package version 3.0.6
-
- Barton K (2019) Multi-modal inference - R Package ‘MuMIn.’ R Packag. version 1
-
- Bruns V. Peripheral auditory tuning for fine frequency analysis by the CF-FM bat, Rhinolophus ferrumequinum - I Mechanical specializations of the cochlea. J Comp Physiol A. 1976;106:77–86. doi: 10.1007/BF00606573. - DOI
-
- Burnhan KP, Anderson DR. Model selection and multimodel inference. New York: Springer; 2002.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

