A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER)
- PMID: 32909660
- PMCID: PMC7756263
- DOI: 10.1111/cea.13731
A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER)
Abstract
Background: The anti-interleukin 13 (IL-13) monoclonal antibody lebrikizumab improves lung function in patients with moderate-to-severe uncontrolled asthma, but its effects on airway inflammation and remodelling are unknown. CLAVIER was designed to assess lebrikizumab's effect on eosinophilic inflammation and remodelling.
Objective: To report safety and efficacy results from enrolled participants with available data from CLAVIER.
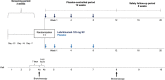
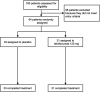
Methods: We performed bronchoscopy on patients with uncontrolled asthma before and after 12 weeks of randomized double-blinded treatment with lebrikizumab (n = 31) or placebo (n = 33). The pre-specified primary end-point was relative change in airway subepithelial eosinophils per mm2 of basement membrane (cells/mm2 ). Pre-specified secondary and exploratory outcomes included change in IL-13-associated biomarkers and measures of airway remodelling.
Results: There was a baseline imbalance in tissue eosinophils and high variability between treatment groups. There was no discernible change in adjusted mean subepithelial eosinophils/mm2 in response to lebrikizumab (95% CI, -82.5%, 97.5%). As previously observed, FEV1 increased after lebrikizumab treatment. Moreover, subepithelial collagen thickness decreased 21.5% after lebrikizumab treatment (95% CI, -32.9%, -10.2%), and fractional exhaled nitric oxide, CCL26 and SERPINB2 mRNA expression in bronchial tissues also reduced. Lebrikizumab was well tolerated, with a safety profile consistent with other lebrikizumab asthma studies.
Conclusions & clinical relevance: We did not observe reduced tissue eosinophil numbers in association with lebrikizumab treatment. However, in pre-specified exploratory analyses, lebrikizumab treatment was associated with reduced degree of subepithelial fibrosis, a feature of airway remodelling, as well as improved lung function and reduced key pharmacodynamic biomarkers in bronchial tissues. These results reinforce the importance of IL-13 in airway pathobiology and suggest that neutralization of IL-13 may reduce asthmatic airway remodelling.
Clinical trial registration: NCT02099656.
© 2020 The Authors. Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors report support of the parent study and funding of editorial support from F. Hoffmann‐La Roche. CDA, MGE, RF, MS, MB, KM, JA, DC, JO, FA, KP, MH, KW, FC, WSP and CTJH are employees of Genentech, Inc. JGM was an employee at Genentech, Inc, at the time of the study but is now an employee of 23andMe. PB has received grant funding from Genentech, Inc. GMG has received research funding from Genentech, Inc, paid directly to McMaster University. MF has received research funding from Genentech, Inc, paid directly to UBC. EI has received grants and nonfinancial support from Genentech during the conduct of the study. MK receives research funding for asthma (paid to the University of Arizona) from the National Institutes of Health, American Lung Association, AstraZeneca and Sanofi. MK engages in consulting activities with AstraZeneca and Sanofi and receives royalties from Elsevier. AB has received personal fees and nonfinancial support from Roche outside the submitted work. PW reports fees to his institution for assistance with analysis of samples in this clinical study and personal fees from Amgen, NGM biopharmaceuticals, Theravance, Clarus Ventures, AstraZeneca, 23andMe, Sanofi, Regeneron and GSK outside the submitted work.
Figures


References
-
- Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S‐12S. - PubMed
-
- Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804‐813. - PubMed
-
- Global Initiative for Asthma . 2019 GINA Report, Global Strategy for Asthma Management and Prevention, 2019.
-
- Lemiere C, Ernst P, Olivenstein R, et al. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and none‐osinophilic phenotypes. J Allergy Clin Immunol. 2006;118(5):1033‐1039. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

