Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment
- PMID: 32910170
- PMCID: PMC7489392
- DOI: 10.1001/jamaoncol.2020.3537
Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment
Abstract
Importance: The benefit of adjuvant chemotherapy after resection of pancreatic cancer following neoadjuvant combination treatment with folinic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) is unclear.
Objective: To assess the association of adjuvant chemotherapy with overall survival (OS) in patients after pancreatic cancer resection and neoadjuvant FOLFIRINOX treatment.
Design, setting, and participants: This international, multicenter, retrospective cohort study was conducted from January 1, 2012, to December 31, 2018. An existing cohort of patients undergoing resection of pancreatic cancer after FOLFIRINOX was updated and expanded for the purpose of this study. All consecutive patients who underwent pancreatic surgery after at least 2 cycles of neoadjuvant FOLFIRINOX chemotherapy for nonmetastatic pancreatic cancer were retrospectively identified from institutional databases. Patients with resectable pancreatic cancer, borderline resectable pancreatic cancer, and locally advanced pancreatic cancer were eligible for this study. Patients with in-hospital mortality or who died within 3 months after surgery were excluded.
Exposures: The association of adjuvant chemotherapy with OS was evaluated in different subgroups including interaction terms for clinicopathological parameters with adjuvant treatment in a multivariable Cox model. Overall survival was defined as the time starting from surgery plus 3 months (moment eligible for adjuvant therapy), unless mentioned otherwise.
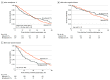
Results: We included 520 patients (median [interquartile range] age, 61 [53-66] years; 279 [53.7%] men) from 31 centers in 19 countries. The median number of neoadjuvant cycles of FOLFIRINOX was 6 (interquartile range, 5-8). Overall, 343 patients (66.0%) received adjuvant chemotherapy, of whom 68 (19.8%) received FOLFIRINOX, 201 (58.6%) received gemcitabine-based chemotherapy, 14 (4.1%) received capecitabine, 45 (13.1%) received a combination or other agents, and 15 (4.4%) received an unknown type of adjuvant chemotherapy. Median OS was 38 months (95% CI, 36-46 months) after diagnosis and 31 months (95% CI, 29-37 months) after surgery. No survival difference was found for patients who received adjuvant chemotherapy vs those who did not (median OS, 29 vs 29 months, univariable hazard ratio [HR], 0.99; 95% CI, 0.77-1.28; P = .93). In multivariable analysis, only the interaction term for lymph node stage with adjuvant therapy was statistically significant: In patients with pathology-proven node-positive disease, adjuvant chemotherapy was associated with improved survival (median OS, 26 vs 13 months; multivariable HR, 0.41 [95% CI, 0.22-0.75]; P = .004). In patients with node-negative disease, adjuvant chemotherapy was not associated with improved survival (median OS, 38 vs 54 months; multivariable HR, 0.85; 95% CI, 0.35-2.10; P = .73).
Conclusions and relevance: These results suggest that adjuvant chemotherapy after neoadjuvant FOLFIRINOX and resection of pancreatic cancer was associated with improved survival only in patients with pathology-proven node-positive disease. Future randomized studies should be conducted to confirm this finding.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical