SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors
- PMID: 32910175
- PMCID: PMC7499592
- DOI: 10.1093/infdis/jiaa581
SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors
Abstract
Background: Convalescent plasma therapy is a leading treatment for conferring temporary immunity to COVID-19-susceptible individuals or for use as post-exposure prophylaxis. However, not all recovered patients develop adequate antibody titers for donation and the relationship between avidity and neutralizing titers is currently not well understood.
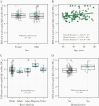
Methods: SARS-CoV-2 anti-spike and anti-nucleocapsid IgG titers and avidity were measured in a longitudinal cohort of COVID-19 hospitalized patients (n = 16 individuals) and a cross-sectional sample of convalescent plasma donors (n = 130). Epidemiologic correlates of avidity were examined in donors by linear regression. The association of avidity and a high neutralizing titer (NT) were also assessed in donors using modified Poisson regression.
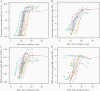
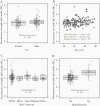
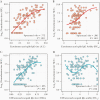
Results: Antibody avidity increased over duration of infection and remained elevated. In convalescent plasma donors, higher levels of anti-spike avidity were associated with older age, male sex, and hospitalization. Higher NTs had a stronger positive correlation with anti-spike IgG avidity (Spearman ρ = 0.386; P < .001) than with anti-nucleocapsid IgG avidity (Spearman ρ = 0.211; P = .026). Increasing levels of anti-spike IgG avidity were associated with high NT (≥160) (adjusted prevalence ratio = 1.58 [95% confidence interval = 1.19-2.12]), independent of age, sex, and hospitalization.
Conclusions: SARS-CoV-2 antibody avidity correlated with duration of infection and higher neutralizing titers, suggesting a potential alternative screening parameter for identifying optimal convalescent plasma donors.
Keywords: SARS-CoV-2; anti-nucleocapsid; anti-spike; avidity; convalescent plasma.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
The effect of early COVID-19 treatment with convalescent plasma on antibody responses to SARS-CoV-2.Microbiol Spectr. 2025 Jul;13(7):e0300624. doi: 10.1128/spectrum.03006-24. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488473 Free PMC article. Clinical Trial.
-
Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization.J Clin Invest. 2020 Dec 1;130(12):6728-6738. doi: 10.1172/JCI141206. J Clin Invest. 2020. PMID: 32910806 Free PMC article. Clinical Trial.
-
Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program.Transfusion. 2020 Dec;60(12):2938-2951. doi: 10.1111/trf.16065. Epub 2020 Sep 16. Transfusion. 2020. PMID: 32935877 Free PMC article.
-
Antibody Responses in COVID-19: A Review.Front Immunol. 2021 Apr 15;12:633184. doi: 10.3389/fimmu.2021.633184. eCollection 2021. Front Immunol. 2021. PMID: 33936045 Free PMC article. Review.
-
Fresh frozen plasma for neutralizing SARS-CoV-2: "An exploratory cross-sectional study and review of the state of the art".Enferm Infecc Microbiol Clin (Engl Ed). 2025 May;43(5):282-290. doi: 10.1016/j.eimce.2025.03.014. Enferm Infecc Microbiol Clin (Engl Ed). 2025. PMID: 40340037 Review.
Cited by
-
Differential antibody production by symptomatology in SARS-CoV-2 convalescent individuals.PLoS One. 2022 Jun 9;17(6):e0264298. doi: 10.1371/journal.pone.0264298. eCollection 2022. PLoS One. 2022. PMID: 35679259 Free PMC article.
-
BNT162b2 mRNA SARS-CoV-2 Vaccine Elicits High Avidity and Neutralizing Antibodies in Healthcare Workers.Vaccines (Basel). 2021 Jun 18;9(6):672. doi: 10.3390/vaccines9060672. Vaccines (Basel). 2021. PMID: 34207300 Free PMC article.
-
Immunotherapy Summary for Cytokine Storm in COVID-19.Front Pharmacol. 2021 Sep 17;12:731847. doi: 10.3389/fphar.2021.731847. eCollection 2021. Front Pharmacol. 2021. PMID: 34603047 Free PMC article. Review.
-
Convalescent plasma for COVID-19 - encouraging signals of efficacy.Br J Haematol. 2021 Feb;192(4):681-682. doi: 10.1111/bjh.17270. Epub 2021 Feb 1. Br J Haematol. 2021. PMID: 33524158 Free PMC article. No abstract available.
-
Low quantity and quality of anti-spike humoral response is linked to CD4 T-cell apoptosis in COVID-19 patients.Cell Death Dis. 2022 Aug 27;13(8):741. doi: 10.1038/s41419-022-05190-0. Cell Death Dis. 2022. PMID: 36030261 Free PMC article.
References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 1 July 2020.
-
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed 28 June 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

