Zn2+ to probe voltage-gated proton (Hv1) channels
- PMID: 32910187
- PMCID: PMC7537345
- DOI: 10.1085/jgp.202012725
Zn2+ to probe voltage-gated proton (Hv1) channels
Abstract
Cherny and coworkers use zinc ion as a probe to identify different conformational states of voltage-gated proton (Hv1) channels.
Figures
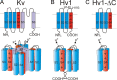
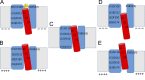
Comment on
-
Engineered high-affinity zinc binding site reveals gating configurations of a human proton channel.J Gen Physiol. 2020 Oct 5;152(10):e202012664. doi: 10.1085/jgp.202012664. J Gen Physiol. 2020. PMID: 32902579 Free PMC article.
Similar articles
-
Molecular mechanism of Zn2+ inhibition of a voltage-gated proton channel.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):E5962-E5971. doi: 10.1073/pnas.1604082113. Epub 2016 Sep 19. Proc Natl Acad Sci U S A. 2016. PMID: 27647906 Free PMC article.
-
Engineered high-affinity zinc binding site reveals gating configurations of a human proton channel.J Gen Physiol. 2020 Oct 5;152(10):e202012664. doi: 10.1085/jgp.202012664. J Gen Physiol. 2020. PMID: 32902579 Free PMC article.
-
New 'kids' on the voltage-gated proton channel block.FEBS J. 2023 Feb;290(4):970-973. doi: 10.1111/febs.16670. Epub 2022 Oct 31. FEBS J. 2023. PMID: 36315610
-
Voltage-gated proton (H(v)1) channels, a singular voltage sensing domain.FEBS Lett. 2015 Nov 14;589(22):3471-8. doi: 10.1016/j.febslet.2015.08.003. Epub 2015 Aug 18. FEBS Lett. 2015. PMID: 26296320 Review.
-
Diversity of voltage gated proton channels.Front Biosci. 2003 Sep 1;8:s1266-79. doi: 10.2741/1191. Front Biosci. 2003. PMID: 12957841 Review.
Cited by
-
The role of Phe150 in human voltage-gated proton channel.iScience. 2022 Oct 20;25(11):105420. doi: 10.1016/j.isci.2022.105420. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388967 Free PMC article.

