Renin-Angiotensin System Inhibitors and COVID-19: a Systematic Review and Meta-Analysis. Evidence for Significant Geographical Disparities
- PMID: 32910274
- PMCID: PMC7481766
- DOI: 10.1007/s11906-020-01101-w
Renin-Angiotensin System Inhibitors and COVID-19: a Systematic Review and Meta-Analysis. Evidence for Significant Geographical Disparities
Abstract
Purpose of review: While the COVID-19 pandemic is constantly evolving, it remains unclear whether the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) affects the clinical course of SARS-CoV-2 infection. For this meta-analysis, PubMed, CENTRAL, and grey literature were searched from their inception to 19 May 2020 for randomized, controlled trials or observational studies that evaluate the association between the use of either ACE inhibitors or ARBs and the risk for major clinical endpoints (infection, hospitalization, admission to ICU, death) in adult patients during the COVID-19 pandemic. In addition, a subgroup geographical analysis of outcomes was performed. Studies including less than 100 subjects were excluded from our analysis.
Recent findings: In total, 25 observational studies were included. ACE inhibitors and ARBs were not associated with increased odds for SARS-CoV-2 infection, admission to hospital, severe or critical illness, admission to ICU, and SARS-CoV-2-related death. In Asian countries, the use of ACE inhibitors/ARBs decreased the odds for severe or critical illness and death (OR = 0.37, 95% CI 0.16-0.89, I2 = 83%, and OR = 0.62, 95% CI 0.39-0.99, I2 = 0%, respectively), whereas they increased the odds for ICU admission in North America and death in Europe (OR = 1.75, 95% CI 1.37-2.23, I2 = 0%, and OR = 1.68, 95% CI 1.05-2.70, I2 = 82%, respectively). ACE inhibitors might be marginally protective regarding SARS-CoV-2-related death compared with ARBs (OR = 0.86, 95% CI 0.74-1.00, I2 = 0%). Randomized controlled trials are needed to confirm the aforementioned associations between ACE inhibitors, ARBs, and SARS-CoV-2.
Keywords: ACE inhibitors; ARBs; Angiotensin receptor blockers; Angiotensin-converting enzyme inhibitors; COVID-19; Hypertension; RAS inhibitors; Renin-angiotensin inhibitors; SARS-CoV-2.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



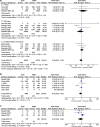
Similar articles
-
A systematic review and meta-analysis of the use of renin-angiotensin system drugs and COVID-19 clinical outcomes: What is the evidence so far?Pharmacol Res Perspect. 2020 Dec;8(6):e00666. doi: 10.1002/prp2.666. Pharmacol Res Perspect. 2020. PMID: 33084232 Free PMC article.
-
Renin-Angiotensin System Blockers and Adverse Outcomes of Influenza and Pneumonia: A Danish Cohort Study.J Am Heart Assoc. 2020 Oct 20;9(19):e017297. doi: 10.1161/JAHA.120.017297. Epub 2020 Oct 1. J Am Heart Assoc. 2020. PMID: 32998607 Free PMC article.
-
Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients.Elife. 2020 Apr 6;9:e57278. doi: 10.7554/eLife.57278. Elife. 2020. PMID: 32250244 Free PMC article.
-
Is the use of ACE inb/ARBs associated with higher in-hospital mortality in Covid-19 pneumonia patients?Clin Exp Hypertens. 2020 Nov 16;42(8):738-742. doi: 10.1080/10641963.2020.1783549. Epub 2020 Jun 22. Clin Exp Hypertens. 2020. PMID: 32569491
-
Outcomes of renin-angiotensin-aldosterone system blockers in patients with COVID-19: a systematic review and meta-analysis.Eur Heart J Cardiovasc Pharmacother. 2020 Sep 1;6(5):335-337. doi: 10.1093/ehjcvp/pvaa074. Eur Heart J Cardiovasc Pharmacother. 2020. PMID: 32671399 Free PMC article. No abstract available.
Cited by
-
Mortality in Patients with COVID-19 on Renin Angiotensin System Inhibitor Long-Term Treatment: An Observational Study Showing that Things Are Not Always as They Seem.Adv Ther. 2021 May;38(5):2709-2716. doi: 10.1007/s12325-021-01704-y. Epub 2021 Apr 1. Adv Ther. 2021. PMID: 33792889 Free PMC article.
-
The age again in the eye of the COVID-19 storm: evidence-based decision making.Immun Ageing. 2021 May 20;18(1):24. doi: 10.1186/s12979-021-00237-w. Immun Ageing. 2021. PMID: 34016150 Free PMC article.
-
Renin-angiotensin system blocker and the COVID-19 aggravation in patients with hypertension, diabetes, renal failure, Cerebro-cardiovascular disease, or pulmonary disease: Report by the COVID-19 Registry Japan.J Cardiol. 2022 Oct;80(4):292-297. doi: 10.1016/j.jjcc.2022.04.001. Epub 2022 Apr 8. J Cardiol. 2022. PMID: 35469713 Free PMC article.
-
Association of outpatient use of renin-angiotensin-aldosterone system blockers on outcomes of acute respiratory illness during the COVID-19 pandemic: a cohort study.BMJ Open. 2022 Jul 6;12(7):e060305. doi: 10.1136/bmjopen-2021-060305. BMJ Open. 2022. PMID: 35793915 Free PMC article.
-
Do COVID-19 CT features vary between patients from within and outside mainland China? Findings from a meta-analysis.Front Public Health. 2022 Oct 14;10:939095. doi: 10.3389/fpubh.2022.939095. eCollection 2022. Front Public Health. 2022. PMID: 36311632 Free PMC article.
References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization, Assessed on May 27th, 2020. https://covid19.who.int/. Accessed 27 May 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

