Comparison of four commercial SARS-CoV-2 IgG immuno-assays in RT-PCR negative patients with suspect CT findings
- PMID: 32910322
- PMCID: PMC7482374
- DOI: 10.1007/s15010-020-01523-3
Comparison of four commercial SARS-CoV-2 IgG immuno-assays in RT-PCR negative patients with suspect CT findings
Abstract
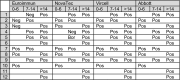
A subset of patients with Covid-19 presents with negative RT-PCR screening but suspect CT findings. Using four commercially available anti-SARS-CoV-2 IgG immuno-assays, we found this subset constituted 9.2% of all consecutively admitted outpatients with Covid-19 in our hospital. Clinical specificity for Covid-19 of some N protein-based immuno-assays was suboptimal, as positive results were observed in control patients with recent common human coronavirus, influenza B and adenovirus infections.
Keywords: Covid-19; ELISA; SARS-CoV-2; Serology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Steensels D, Reynders M, Descheemaeker P, Curran MD, Jacobs F, Denis O, et al. Performance evaluation of direct fluorescent antibody, Focus Diagnostics Simplexa Flu A/B and RSV and multi-parameter customized respiratory Taqman(R) array card in immunocompromised patients. J Virol Methods. 2017;245:61–65. doi: 10.1016/j.jviromet.2017.03.013. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

