Comparative Antiviral Efficacy of Viral Protease Inhibitors against the Novel SARS-CoV-2 In Vitro
- PMID: 32910347
- PMCID: PMC7481761
- DOI: 10.1007/s12250-020-00288-1
Comparative Antiviral Efficacy of Viral Protease Inhibitors against the Novel SARS-CoV-2 In Vitro
Abstract
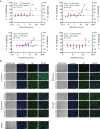
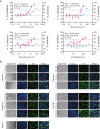
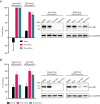
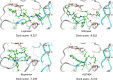
The recent outbreak of novel coronavirus pneumonia (COVID-19) caused by a new coronavirus has posed a great threat to public health. Identifying safe and effective antivirals is of urgent demand to cure the huge number of patients. Virus-encoded proteases are considered potential drug targets. The human immunodeficiency virus protease inhibitors (lopinavir/ritonavir) has been recommended in the global Solidarity Trial in March launched by World Health Organization. However, there is currently no experimental evidence to support or against its clinical use. We evaluated the antiviral efficacy of lopinavir/ritonavir along with other two viral protease inhibitors in vitro, and discussed the possible inhibitory mechanism in silico. The in vitro to in vivo extrapolation was carried out to assess whether lopinavir/ritonavir could be effective in clinical. Among the four tested compounds, lopinavir showed the best inhibitory effect against the novel coronavirus infection. However, further in vitro to in vivo extrapolation of pharmacokinetics suggested that lopinavir/ritonavir could not reach effective concentration under standard dosing regimen [marketed as Kaletra®, contained lopinavir/ritonavir (200 mg/50 mg) tablets, recommended dosage is 400 mg/10 mg (2 tablets) twice daily]. This research concluded that lopinavir/ritonavir should be stopped for clinical use due to the huge gap between in vitro IC50 and free plasma concentration. Nevertheless, the structure-activity relationship analysis of the four inhibitors provided further information for de novel design of future viral protease inhibitors of SARS-CoV-2.
Keywords: COVID-19; Protease inhibitor; Respiratory pharmacology; SARS-CoV-2.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. - DOI - PMC - PubMed
-
- Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

