The Challenges of Switching Therapies in an Evolving Multiple Biosimilars Landscape: A Narrative Review of Current Evidence
- PMID: 32910420
- PMCID: PMC7547992
- DOI: 10.1007/s12325-020-01472-1
The Challenges of Switching Therapies in an Evolving Multiple Biosimilars Landscape: A Narrative Review of Current Evidence
Abstract
With the increasing availability of biosimilars, the practice of switching therapies for non-medical reasons between an originator biologic and an analogous biosimilar has become more common. The evidence to support this practice mostly comes from single-switch randomized controlled trials (RCTs) and real-world (RW) evidence studies. However, as more biosimilars of the same originator enter the market, multiple switching events between originators and biosimilars is becoming a reality, despite limited evidence to support the efficacy and safety of such practice. Some countries have established guidelines, policies, or laws related to interchangeability and/or automatic substitution, whereas others have left these practices unregulated or controlled by other components of the healthcare system. Collectively, guidelines on single non-medical switching are often vague, with even less focus given to multiple non-medical switching, leaving this practice mostly unregulated. This narrative review will first discuss the current regulatory perspectives on non-medical switching and challenges associated with switching therapies, particularly with the availability of multiple biosimilars. We will then review the current evidence from RCTs and RW studies in the light of three different multiple-switch scenarios currently taking place in clinical practice: switching between an originator and a single biosimilar, switching between biosimilars of the same originator, and the clinical practice of switching back to the originator (i.e., switchbacks) after a failure of the initial non-medical switch to the analogous biosimilar.
Keywords: Biosimilar; Multiple switch; Non-medical switching; Originator; Switchback.
Figures
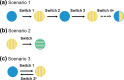
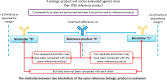
Comment in
-
Letter to the Editor Regarding "The Challenges of Switching Therapies in an Evolving Multiple Biosimilars Landscape: A Narrative Review of Current Evidence".Adv Ther. 2021 Jun;38(6):3483-3486. doi: 10.1007/s12325-021-01694-x. Epub 2021 Apr 29. Adv Ther. 2021. PMID: 33914268 Free PMC article. No abstract available.
References
-
- Yifei L, Skup M, Yang M, Qi C, Doctor T. Prevalence rates of biosimilar discontinuation and switchback to originator biologics following non-medical switching: a meta-analysis of real-world studies [abstract P445] J Crohns Colitis. 2019;13:S334.
-
- Reiland J-B, Freischem B, Roediger A. What pricing and reimbursement policies to use for off-patent biologicals in Europe? Results from the second EBE biological medicines policy survey. GaBI J. 2017;6:1–18.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

