The Skin Microbiome: A New Actor in Inflammatory Acne
- PMID: 32910436
- PMCID: PMC7584556
- DOI: 10.1007/s40257-020-00531-1
The Skin Microbiome: A New Actor in Inflammatory Acne
Abstract
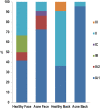
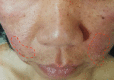
Our understanding of the role of Cutibacterium acnes in the pathophysiology of acne has recently undergone a paradigm shift: rather than C. acnes hyperproliferation, it is the loss of balance between the different C. acnes phylotypes, together with a dysbiosis of the skin microbiome, which results in acne development. The loss of diversity of C. acnes phylotypes acts as a trigger for innate immune system activation, leading to cutaneous inflammation. A predominance of C. acnes phylotype IA1 has been observed, with a more virulent profile in acne than in normal skin. Other bacteria, mainly Staphylococcus epidermis, are also implicated in acne. S. epidermidis and C. acnes interact and are critical for the regulation of skin homeostasis. Recent studies also showed that the gut microbiome is involved in acne, through interactions with the skin microbiome. As commonly used topical and systemic antibiotics induce cutaneous dysbiosis, our new understanding of acne pathophysiology has prompted a change in direction for acne treatment. In the future, the development of individualized acne therapies will allow targeting of the pathogenic strains, leaving the commensal strains intact. Such alternative treatments, involving modifications of the microbiome, will form the next generation of 'ecobiological' anti-inflammatory treatments.
Conflict of interest statement
No conflict to be declared on this topic.
Figures
References
-
- Suh DH, Kwon HH. What’s new in the physiopathology of acne? Br J Dermatol. 2015;172(Suppl 1):13–19. - PubMed
-
- Dreno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(Suppl 5):8–12. - PubMed
-
- Hall JB, Cong Z, Imamura-Kawasawa Y, Kidd BA, Dudley JT, Thiboutot DM, et al. Isolation and identification of the follicular microbiome: implications for Acne research. J Invest Dermatol. 2018;138(9):2033–2040. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

