Tofacitinib in Patients With Psoriatic Arthritis and Metabolic Syndrome: A Post hoc Analysis of Phase 3 Studies
- PMID: 32910531
- PMCID: PMC7571390
- DOI: 10.1002/acr2.11166
Tofacitinib in Patients With Psoriatic Arthritis and Metabolic Syndrome: A Post hoc Analysis of Phase 3 Studies
Abstract
Objective: Metabolic syndrome (MetS) is a cluster of concurrent risk factors for cardiovascular disease and type 2 diabetes. This post hoc analysis explored key efficacy and safety endpoints in patients with psoriatic arthritis (PsA) and MetS treated with tofacitinib.
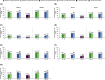
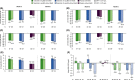
Methods: Tofacitinib 5 and 10 mg twice daily and placebo data were pooled from two Phase 3 studies (OPAL Broaden [12 months; ClinicalTrials.gov identifier NCT01877668]; OPAL Beyond [6 months; ClinicalTrials.gov identifier NCT01882439]); patients received one background conventional synthetic disease-modifying antirheumatic drug. Patients were stratified by baseline presence/absence of MetS. Efficacy and safety were reported to month 3 (tofacitinib and placebo) and 6 (tofacitinib only). Efficacy outcomes included: American College of Rheumatology (ACR)20/50/70, Health Assessment Questionnaire-Disability Index (HAQ-DI) response, Psoriasis Area Severity Index (PASI)75 response, and enthesitis/dactylitis resolution rates; and changes from baseline (Δ) in C-reactive protein, HAQ-DI, Patient's/Physician's Global Assessment of Arthritis, and patient-reported outcomes. Safety outcomes included treatment-emergent all-causality adverse events (AEs), Δ in lipid/hepatic values, and liver parameter increases.
Results: Of 710 patients, 41.4% (n = 294) had baseline MetS. All efficacy outcomes improved with both tofacitinib doses versus placebo, to month 3; tofacitinib efficacy was consistent to month 6, regardless of MetS status. MetS did not appear to affect the incidence of AEs or Δ in lipid/hepatic values with tofacitinib up to month 3 or 6. Arterial thromboembolism and myocardial infarction (adjudicated major adverse cardiovascular events) were each reported once in tofacitinib-treated patients with MetS.
Conclusion: Regardless of baseline MetS status, tofacitinib showed greater efficacy versus placebo in patients with active PsA. The tofacitinib safety profile appeared similar in patients with versus without MetS.
© 2020 The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
Similar articles
-
Efficacy and safety of tofacitinib by background methotrexate dose in psoriatic arthritis: post hoc exploratory analysis from two phase III trials.Clin Rheumatol. 2022 Feb;41(2):499-511. doi: 10.1007/s10067-021-05894-2. Epub 2021 Sep 12. Clin Rheumatol. 2022. PMID: 34510295 Free PMC article. Clinical Trial.
-
Efficacy of Tofacitinib for the Treatment of Psoriatic Arthritis: Pooled Analysis of Two Phase 3 Studies.Rheumatol Ther. 2018 Dec;5(2):567-582. doi: 10.1007/s40744-018-0131-5. Epub 2018 Nov 9. Rheumatol Ther. 2018. PMID: 30414064 Free PMC article.
-
Safety and Efficacy of Tofacitinib in Patients with Active Psoriatic Arthritis: Interim Analysis of OPAL Balance, an Open-Label, Long-Term Extension Study.Rheumatol Ther. 2020 Sep;7(3):553-580. doi: 10.1007/s40744-020-00209-4. Epub 2020 Jun 6. Rheumatol Ther. 2020. PMID: 32506317 Free PMC article.
-
Efficacy and safety of tofacitinib for chronic plaque psoriasis and psoriatic arthritis: a systematic review and meta-analysis of randomized controlled trials.Clin Rheumatol. 2024 May;43(5):1605-1613. doi: 10.1007/s10067-024-06940-5. Epub 2024 Mar 22. Clin Rheumatol. 2024. PMID: 38517652
-
Tofacitinib 5 mg Twice Daily in Patients with Rheumatoid Arthritis and Inadequate Response to Disease-Modifying Antirheumatic Drugs: A Comprehensive Review of Phase 3 Efficacy and Safety.J Clin Rheumatol. 2019 Apr;25(3):115-126. doi: 10.1097/RHU.0000000000000786. J Clin Rheumatol. 2019. PMID: 29794874 Free PMC article. Review.
Cited by
-
Psoriatic Arthritis and Metabolic Syndrome: Is There a Role for Disease Modifying Anti-Rheumatic Drugs?Front Med (Lausanne). 2021 Aug 30;8:735150. doi: 10.3389/fmed.2021.735150. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34527685 Free PMC article. Review.
-
Anti- and non-tumor necrosis factor-α-targeted therapies effects on insulin resistance in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis.World J Diabetes. 2021 Mar 15;12(3):238-260. doi: 10.4239/wjd.v12.i3.238. World J Diabetes. 2021. PMID: 33758645 Free PMC article. Review.
-
Risk Stratification of Patients with Psoriatic Arthritis and Ankylosing Spondylitis for Treatment with Tofacitinib: A Review of Current Clinical Data.Rheumatol Ther. 2024 Jun;11(3):487-499. doi: 10.1007/s40744-024-00662-5. Epub 2024 May 2. Rheumatol Ther. 2024. PMID: 38696034 Free PMC article.
-
Choosing the Appropriate Target for the Treatment of Psoriatic Arthritis: TNFα, IL-17, IL-23 or JAK Inhibitors?Mediterr J Rheumatol. 2022 Apr 15;33(Suppl 1):150-161. doi: 10.31138/mjr.33.1.150. eCollection 2022 Mar. Mediterr J Rheumatol. 2022. PMID: 36127928 Free PMC article. Review.
References
-
- Haroon M, Gallagher P, Heffernan E, FitzGerald O. High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol 2014;41:1357–65. - PubMed
-
- Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. - PubMed
-
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta‐analysis. J Am Coll Cardiol 2010;56:1113–32. - PubMed
-
- Meigs JB, Wilson PWF, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006;91:2906–12. - PubMed
-
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004;15:2792–800. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

