Liver epithelial focal adhesion kinase modulates fibrogenesis and hedgehog signaling
- PMID: 32910808
- PMCID: PMC7605528
- DOI: 10.1172/jci.insight.141217
Liver epithelial focal adhesion kinase modulates fibrogenesis and hedgehog signaling
Abstract
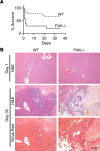
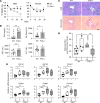
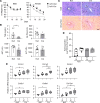
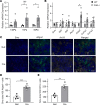
Focal adhesion kinase (FAK) is an important mediator of extracellular matrix-integrin mechano-signal transduction that regulates cell motility, survival, and proliferation. As such, FAK is being investigated as a potential therapeutic target for malignant and fibrotic diseases, and numerous clinical trials of FAK inhibitors are underway. The function of FAK in nonmalignant, nonmotile epithelial cells is not well understood. We previously showed that hepatocytes demonstrated activated FAK near stiff collagen tracts in fibrotic livers. In this study, we examined the role of liver epithelial FAK by inducing fibrotic liver disease in mice with liver epithelial FAK deficiency. We found that mice that lacked FAK in liver epithelial cells developed more severe liver injury and worse fibrosis as compared with controls. Increased fibrosis in liver epithelial FAK-deficient mice was linked to the activation of several profibrotic pathways, including the hedgehog/smoothened pathway. FAK-deficient hepatocytes produced increased Indian hedgehog in a manner dependent on matrix stiffness. Furthermore, expression of the hedgehog receptor, smoothened, was increased in macrophages and biliary cells of hepatocyte-specific FAK-deficient fibrotic livers. These results indicate that liver epithelial FAK has important regulatory roles in the response to liver injury and progression of fibrosis.
Keywords: Cell Biology; Fibrosis; Hepatology; Integrins; Signal transduction.
Conflict of interest statement
Figures

Similar articles
-
Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells.Gastroenterology. 2018 Apr;154(5):1465-1479.e13. doi: 10.1053/j.gastro.2017.12.022. Epub 2018 Jan 3. Gastroenterology. 2018. PMID: 29305935 Free PMC article.
-
MicroRNA-378 is involved in hedgehog-driven epithelial-to-mesenchymal transition in hepatocytes of regenerating liver.Cell Death Dis. 2018 Jun 18;9(7):721. doi: 10.1038/s41419-018-0762-z. Cell Death Dis. 2018. PMID: 29915286 Free PMC article.
-
The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury.J Hepatol. 2014 Jan;60(1):143-51. doi: 10.1016/j.jhep.2013.08.012. Epub 2013 Aug 23. J Hepatol. 2014. PMID: 23978713
-
Signal transduction by focal adhesion kinase in cancer.Cancer Metastasis Rev. 2009 Jun;28(1-2):35-49. doi: 10.1007/s10555-008-9165-4. Cancer Metastasis Rev. 2009. PMID: 19169797 Review.
-
Biochemical signals and biological responses elicited by the focal adhesion kinase.Biochim Biophys Acta. 2001 Jul 25;1540(1):1-21. doi: 10.1016/s0167-4889(01)00123-9. Biochim Biophys Acta. 2001. PMID: 11476890 Review.
Cited by
-
On the Value of In Vitro Cell Systems for Mechanobiology from the Perspective of Yes-Associated Protein/Transcriptional Co-Activator with a PDZ-Binding Motif and Focal Adhesion Kinase and Their Involvement in Wound Healing, Cancer, Aging, and Senescence.Int J Mol Sci. 2023 Aug 11;24(16):12677. doi: 10.3390/ijms241612677. Int J Mol Sci. 2023. PMID: 37628858 Free PMC article. Review.
-
Cullin3 (CUL3) suppresses proliferation, migration and phenotypic transformation of PDGF-BB-stimulated vascular smooth muscle cells and mitigates inflammatory response by repressing Hedgehog signaling pathway.Bioengineered. 2021 Dec;12(2):9463-9472. doi: 10.1080/21655979.2021.1995572. Bioengineered. 2021. PMID: 34699319 Free PMC article.
-
Lactobacillus johnsonii alleviates experimental colitis by restoring intestinal barrier function and reducing NET-mediated gut-liver inflammation.Commun Biol. 2025 Aug 14;8(1):1222. doi: 10.1038/s42003-025-08679-4. Commun Biol. 2025. PMID: 40813467 Free PMC article.
-
Effect of Curcumin on Attenuation of Liver Cirrhosis via Genes/Proteins and Pathways: A System Pharmacology Study.Nutrients. 2022 Oct 17;14(20):4344. doi: 10.3390/nu14204344. Nutrients. 2022. PMID: 36297027 Free PMC article.
-
Stiffness is associated with hepatic stellate cell heterogeneity during liver fibrosis.Am J Physiol Gastrointest Liver Physiol. 2022 Feb 1;322(2):G234-G246. doi: 10.1152/ajpgi.00254.2021. Epub 2021 Dec 23. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 34941452 Free PMC article.
References
-
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692(2-3):103–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

