Inhibition of γ-glutamyltransferase ameliorates ischaemia-reoxygenation tissue damage in rats with hepatic steatosis
- PMID: 32910829
- PMCID: PMC7589019
- DOI: 10.1111/bph.15258
Inhibition of γ-glutamyltransferase ameliorates ischaemia-reoxygenation tissue damage in rats with hepatic steatosis
Abstract
Background and purpose: Hepatic steatosis may be associated with an increased γ-glutamyltransferase (γ-GT) levels. Ischaemia-reoxygenation (IR) injury causes several deleterious effects. We evaluated the protective effects of a selective inhibitor of γ-GT in experimentally induced IR injury in rats with obesity and steatosis.
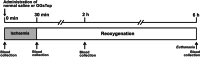
Experimental approach: Otsuka Long-Evans Tokushima Fatty (OLETF) rats with hepatic steatosis were used in the current study. The portal vein and hepatic artery of left lateral and median lobes were clamped to induce ischaemia. Before clamping, 1 ml of saline (IR group) or 1-ml saline containing 1 mg·kg-1 body weight of GGsTop (γ-GT inhibitor; IR-GGsTop group) was injected into the liver via the inferior vena cava. Blood flow was restored after at 30 min of the start of ischaemia. Blood was collected before, at 30 min after ischaemia and at 2 h and 6 h after reoxygenation. All the animals were killed at 6 h and the livers were collected.
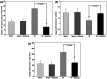
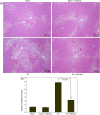
Key results: Treatment with GGsTop resulted in significant reduction of serum ALT, AST and γ-GT levels and hepatic γ-GT, malondialdehyde, 4-hydroxy-2-nonenal and HMGB1 at 6 h after reoxygenation. Inhibition of γ-GT retained normal hepatic glutathione levels. There was prominent hepatic necrosis in IR group, which is significantly reduced in IR-GGsTop group.
Conclusion and implications: Treatment with GGsTop significantly increased hepatic glutathione content, reduced hepatic MDA, 4-HNE and HMGB1 levels and, remarkably, ameliorated hepatic necrosis after ischaemia-reoxygenation. The results indicated that GGsTop could be an appropriate therapeutic agent to reduce IR-induced liver injury in obesity and steatosis.
Keywords: GGsTop; ischaemia; ischaemia-reoxygenation injury; steatosis; γ-glutamyl transpeptidase.
© 2020. The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ahmadvand, H. , Babaeenezhad, E. , Nasri, M. , Jafaripour, L. , & Khorramabadi, R. M. (2019). Glutathione ameliorates liver markers, oxidative stress and inflammatory indices in rats with renal ischemia reperfusion injury. Journal of Renal Injury Prevention, 8(2), 91–97. 10.15171/jrip.2019.18 - DOI
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

