Perspectives on the Classical Enzyme Carbonic Anhydrase and the Search for Inhibitors
- PMID: 32910900
- PMCID: PMC7567974
- DOI: 10.1016/j.bpj.2020.08.020
Perspectives on the Classical Enzyme Carbonic Anhydrase and the Search for Inhibitors
Abstract
Carbonic anhydrase (CA) is a thoroughly studied enzyme. Its primary role is the rapid interconversion of carbon dioxide and bicarbonate in the cells, where carbon dioxide is produced, and in the lungs, where it is released from the blood. At the same time, it regulates pH homeostasis. The inhibitory function of sulfonamides on CA was discovered some 80 years ago. There are numerous physiological-therapeutic conditions in which inhibitors of carbonic anhydrase have a positive effect, such as glaucoma, or act as diuretics. With the realization that several isoenzymes of carbonic anhydrase are associated with the development of several types of cancer, such as brain and breast cancer, the development of inhibitor drugs specific to those enzyme forms has exploded. We would like to highlight the breadth of research on the enzyme as well as draw the attention to some problems in recent published work on inhibitor discovery.
Copyright © 2020 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
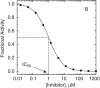
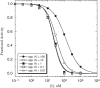

Comment in
-
Response to Perspectives on the Classical Enzyme Carbonic Anhydrase and the Search for Inhibitors.Biophys J. 2021 Jan 5;120(1):178-181. doi: 10.1016/j.bpj.2020.11.011. Epub 2020 Dec 8. Biophys J. 2021. PMID: 33296668 Free PMC article. No abstract available.
-
Comments to the Editor Due to the Response by the Supuran Group to Our Article.Biophys J. 2021 Jan 5;120(1):182-183. doi: 10.1016/j.bpj.2020.11.012. Epub 2020 Dec 13. Biophys J. 2021. PMID: 33308476 Free PMC article. No abstract available.
References
-
- Meldrum N.U., Roughton F.J. Some properties of carbonic anhydrase, the CO2 enzyme present in blood. J. Physiol. 1932;75:15P–16P.
-
- Silverman D.N., Lindskog S. The catalytic mechanism of carbonic anhydrase: implications of a rate limiting protolysis of water. Acc. Chem. Res. 1988;21:30–36.
-
- Pastorekova S., Kopacek J., Pastorek J. Carbonic anhydrase inhibitors and the management of cancer. Curr. Top. Med. Chem. 2007;7:865–878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

